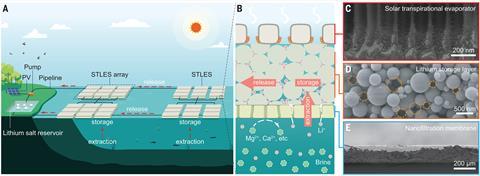
A new solar-powered device harvests lithium from brines while producing almost no greenhouse gases. The membrane-based system could provide a greener way to produce the metal, which is under increasing demand for use in rechargeable batteries.
A team led by Baoxia Mi from the University of California, Berkeley, US and Jia Zhu from Nanjing University in China, developed the floating device having been inspired by the way plants that grow in salty environments obtain key ions from their surroundings. The device features a hierarchically structured membrane system, with separate layers for evaporation, nanofiltration and storage.

This design allows the device to passively extract lithium salts from saline environments in a way that is more efficient in terms of cost, energy, and carbon than conventional lithium-mining techniques.
‘From an environmental perspective, the most exciting aspect of our solar transpiration-powered lithium extraction technology is its complete reliance on sunlight, requiring no external energy inputs,’ says Zhu, ‘This enables truly green and sustainable lithium mining, minimising the environmental footprint.’
A pressure gradient created by solar-driven evaporation draws material into the device, where a specially sized porous membrane allows lithium ions to pass into the storage section of the device, while preventing the uptake of other ions. Water circulation regenerates the device by transferring lithium to a reservoir.
Under ambient outdoor conditions, each module harvests an average of 33.2 milligrams of lithium per square meter each day from brines that contain three milligrams of lithium per litre of water. Arrays of the modules can float on brine ponds that are already used for the majority of lithium mining.
Lithium selectivity is calculated for the device as a ratio of magnesium to lithium inside the device relative to the magnesium to lithium ratio of the feedstock brine. ‘It’s important to note that our solar transpiration–powered lithium extraction technology is compatible with membrane filtration systems,’ says Zhu. ‘We achieved a lithium selectivity of up to 24 using advanced nanofiltration membranes. By employing a staged setup, we were able to increase lithium selectivity to 168. As membrane technology advances, we expect further improvements in the system’s lithium selectivity.’
‘Separating lithium from brine is essential for securing critical materials for the battery supply chain. This study demonstrates a low-energy method of using sunlight to separate lithium,’ comments Menachem Elimelech, expert in environmental and water quality engineering from Yale University in the US.
‘While the method depends on the future development of lithium-selective membranes and significantly increasing the water flux of the membrane, it represents a step forward toward sustainable lithium mining,’ adds Elimelech. ‘A crucial next step in evaluating the practical viability of this technology is a detailed techno-economic analysis comparing its feasibility to conventional methods.’
References
Y Song et al, Science, 2024, DOI: 10.1126/science.adm7034














No comments yet