Supramolecular mask simplifies fullerene modification
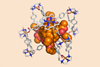
Porphyrin structure makes it easier to perform selective reactions on fullerene substrates
A supramolecular mask makes it easier to perform selective reactions on fullerene compounds. This could help chemists developing solar cell materials to investigate the properties of functionalised C60 molecules.
