Surface reactions can be controlled by bulk stoichiometry
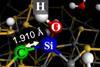
Computational models demonstrate how the elastic properties of a solid can influence surface chemistry
Bulk properties have an unexpected impact on surface reactivity, new research has shown. With applications as wide-ranging as catalysis, energy storage and structural engineering, this new mode of reaction control could have significant implications across the applied sciences.