Surface sites on gold catalyst edited to control regioselectivity of a click reaction
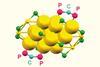
Steric hindrance created by phosphine ligands restricts how substrates approach catalyst
A new study has shown that precisely editing the ligands of a gold nanoparticle catalyst can alter the ratio of products formed by an organic reaction. With such a degree of control, this could prove to be a potent tool in the arsenal of synthetic chemists trying to manipulate increasingly complex reactions in pursuit of new molecules.