Theoretical study predicts iron–carbon quadruple bond
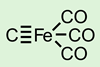
Bond could begin a new trend in main group multiple bonding
Computational research by a team in India predicts that a quadruple bond could form between iron and carbon in the compound C(Fe)(CO)3.