Three centres, two electrons, but how many bonds?
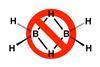
Tweaking Lewis structures allows for a simplified and clearer representation of electron-deficient molecules
Lewis structures are the universal language chemists around the world use to represent molecules. However, some chemists think that these conventions can be misleading. Gerard Parkin from Columbia University in New York, US, is one of those chemists and he has come up with a new approach to representing these structure that he says makes them much clearer. This solution is particularly helpful for representing electron-deficient compounds such as diborane.
