Total synthesis of anti-addictive alkaloid in just seven steps
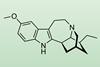
A streamlined synthesis of the psychoactive compound ibogaine could open up new opportunities in the treatment of drug addiction and depression
A short modular synthesis has been developed to produce the psychoactive substance ibogaine and related compounds.1 The approach could help to reduce dependency on natural plant sources for these products and generate new compounds with potential applications in the treatment of drug addiction and depression.