Researchers in China have revealed a new relaxation-related mechanism in a material with negative thermal expansion (NTE). The group introduced organic linkers into the skeleton of a metal–organic rotaxane network to construct a flexible NTE material, which they envision will aid the design of other types of metal–organic materials with controllable thermal responsive behaviour.
Thermal expansion in solid-state materials can be a significant issue, leading to incompatibility and failure in scientific instruments. NTE is an unusual physicochemical process where a material contracts, rather than expands, upon heating. Using NTE materials in composites therefore provides an opportunity to tailor or eliminate undesired thermal expansion.
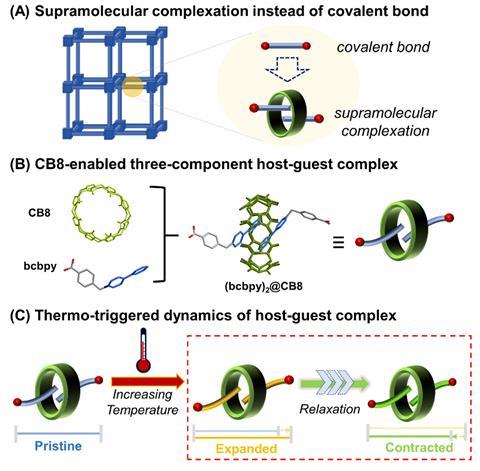
Researchers have already created several NTE materials with a framework structure, including molecular frameworks and metal–organic frameworks (MOFs). In recent years, some teams have incorporated flexible bridging atoms or groups into these frameworks to facilitate stronger NTE. And some have suggested that adding weak interactions into a framework could enhance its flexibility and NTE performance. Now, a team led by Wei-qun Shi, from the Chinese Academy of Sciences, has developed a strategy for designing lattice structures that replaces covalent bonds with noncovalent interactions to improve the thermal response of framework structure materials.
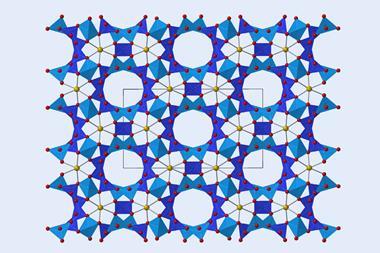
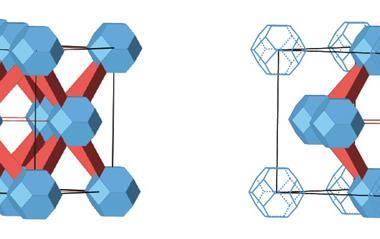
The structure they reported, U3(bcbpy)3(CB8), comprises a uranyl-based metal–organic framework where the organic linkers thread through cucurbit[8]uril (CB8), creating three-component pseudorotaxane units. Involving a supramolecular unit in the framework brings the flexibility ‘to a new level’, comments Shi.
To understand the underlying mechanism for the large NTE effect, the scientists compared crystal structures of U3(bcbpy)3(CB8) obtained at different temperatures. They observed that while the well-defined host–guest inclusion pattern within the cavity of macrocyclic CB8 showed no noticeable changes, the molecular conformation of the V-shaped bcbpy guest connected by the flexible methylene linkage underwent thermally-induced distortion. Crystallographic snapshots of the framework revealed that the terminal bipyridinium motif hinged by the methylene group first experiences a small stretching movement upon heating, followed by a drastic spring-like contraction at 260K.
‘One of the most commonly used tactics for tailoring NTE is to introduce flexibility chemical methods through chemical design and structural construction,’ says Qilong Gao, who researches solid-state chemistry at Zhengzhou University in China. He notes how the study uses ‘a new kind of organic linkage, ie flexible supramolecular complexation’ for the first time to achieve ‘improved NTE performance and structural features’.
The mechanism discovered in this study is ‘totally different’ from the well-known hinging model, explains Shi. In the hinging model reported for other NTE MOFs, a combination of local and collective transverse vibrations correspond to contracting motions of rigid motifs driven by transverse displacement of flexible bridging ligands. While U3(bcbpy)3(CB8) also undergoes transverse vibrations of the flexible bcbpy groups with increasing temperature, the scientists say these are present within the entire temperature range including both the positive thermal expansion and NTE regions. They suggest this is due to a difference in structural flexibility and adaptivity of the weakly-bonded polythreading framework compared with stronger coordination bonds in other MOFs. The unique mechanism they report involves a contraction-related NTE effect corresponding to the recovery stage, which the scientists suggest is a relaxation process after a progressive expansion.
Using uranium in the U3(bcbpy)3(CB8) material may restrict its wider applications. However, the scientists hope that exploring new NTE mechanisms will facilitate the design of other functional MOFs with controllable thermal responsive behaviour.

![[Br4F21]- index image](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/1/7/3/532173_br4f21indeximage_49094.jpg)






No comments yet