Unusual hydrogen bonds found in proteins help them bind their targets
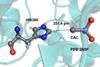
Weak interactions between hydrogen and carbon atoms have synthetic chemistry implications
Chemists in India have published what they say is the first evidence for hydrogen bonds with tetravalent carbon atoms in proteins. Himansu Biswal’s team at the National Institute of Science Education and Research (Niser) in Bhubaneswar found 1051 such non-covalent interactions in existing Protein Data Bank (PDB) crystal structure information.
