Uranium and thorium make their debut in dual aromatic–antiaromatic molecule
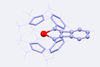
First two metallabiphenylene compounds synthesised
The first metallabiphenylenes – molecules that have both aromatic and antiaromatic rings – have been made by chemists in the US. One of the compounds contains a uranium, the other a thorium atom.
