Researchers have demonstrated that waste gas from the steel industry can be used to prepare pharmaceuticals and fine chemicals.1
Steelmaking is one of the largest carbon-emitting industrial processes in the modern world.2 The principle refining step uses a blast furnace to heat iron ore with carbon, which reduces it to steel and generates waste gas in the process. This so-called converter gas principally consists of carbon monoxide, carbon dioxide and nitrogen. In most production plants the carbon monoxide is reacted with oxygen to generate heat and more carbon dioxide. The resulting mixture of carbon dioxide and nitrogen is then released into the atmosphere.
Now, researchers led by Matthias Beller and Rajenhally Jagadeesh of the Leibniz Institute for Catalysis, Germany, and Denis Chusov of the Nesmeyanov Institute of Organoelement Compounds, Russia, have shown that converter gas can function as a feedstock for key organic transformations. ‘We thought that this waste product could be used for some processes,’ explains Chusov. ‘Carbon monoxide as a reducing agent is very selective as it cannot hydrogenate functional groups. During our research we were pleasantly surprised that the converter gas performed significantly better than expected.’
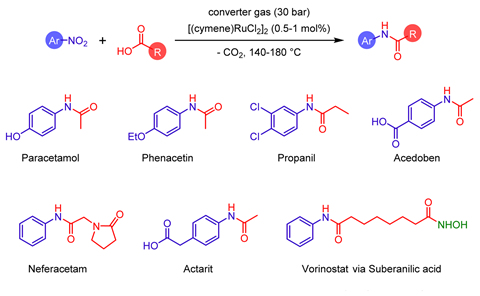
The group initially focused on coupling nitroarenes with carboxylic acids in an amidation reaction using a ruthenium-based catalyst. Performing the reaction under an atmosphere of converter gas led to very high yields of the desired target molecules, including paracetamol and vorinostat, which is used to treat cutaneous T-cell lymphoma. Furthermore, the process was not significantly inhibited by sulfur-based impurities commonly present in the gas.
The same converter gas atmosphere also proved effective in performing an alternative carbon–nitrogen bond forming process through reductive amination with a rhodium catalyst. Antifungal agent butenafine could be prepared in high yield along with a range of other compounds.
The team say that their concept does not require extraordinary safety concerns. Unlike metal or hydride-based reducing agents, it doesn’t generate solid side-products, which can complicate purification.
It’s unclear exactly how the amidation reactions proceed, but the researchers think that the converter gas reacts with the metal catalysts to form highly active metal-carbonyl species that then couple with the reagents to form new carbon–nitrogen bonds. Carbon dioxide in the converter gas also appears to accelerate the reaction.
‘Any time you can take a waste product and turn it into something useful is a really important advance,’ comments Jennifer Love from the University of Calgary, Canada, whose research involves developing new catalytic transformations. ‘Synthesising so many molecules of real-life importance is particularly compelling, and overall I was really excited by this study.’
The team hope that converter gas can be used for further large-scale reductive transformations in the future. ‘In particular, converter gas from steel plants might replace natural gas sources in well-known large-scale industrial processes such as the synthesis of acetic acid and urea,’ adds Chusov.
References
1 S A Runikhina et al, Chem. Sci., 2023, 14, 4346 (DOI: 10.1039/d3sc00257h)
2 M Flores-Granobles and M Saeys, Energy Environ. Sci., 2020, 13, 1923 (DOI: 10.1039/d0ee00787k)










No comments yet