Useful small molecules with potential applications in the cosmetics and fine chemical industries have been recovered from waste plastics by scientists in the US.1
Petroleum based polymers such as polyesters and polycarbonates make up a significant proportion of the 100 million tonnes of plastic waste generated globally every year, of which only between 5–30% is recycled. Traditional recycling commonly leads to new plastics with inferior properties that frequently find use in lower grade applications, such as fibres or carpeting.
A team of undergraduates working under Nicholas Robertson, from Northland College in Wisconsin, and Michael Carney, from the University of Wisconsin-Eau Claire, have developed a way to depolymerise polyesters and polycarbonates into diols and methanol, using ruthenium based pincer catalysts developed by David Milstein and co-workers at the Weizmann Institute of Science in Israel.2 These catalysts have an impressive variety of uses, including polymer synthesis. However, when exposed to high pressure hydrogen gas, the process is reversed and depolymerisation takes place. The pincer catalyst hydrogenates the ester linked backbone of the polymer, unzipping it into small molecules.
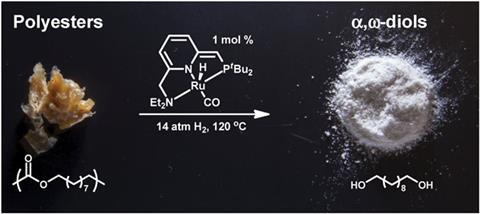
Gretchen Hofmeister, a polymerisation catalysis expert at Carleton College in Minnesota, US, who was not involved in the work, says the advantage of depolymerisation over recycling is that the resultant molecules can be purified before re-use. ‘Without purification, the monomers could not be incorporated into high-performance materials.’
The method is capable of producing small molecules of greater value than the starting monomers, which can be used in various applications such as cosmetics, medicines, and in the manufacture of new high quality polymers.
Robertson says depolymerising plastics into value added chemicals, which are currently more expensive than the monomers from which the polymer is derived, means there is potential for this approach to become cost competitive with petroleum sources. ‘We can potentially start to reduce the flow of petroleum in some of these areas,’ he adds.
‘Chemical recycling is a nice and complementary approach to the ultimate goal of sustainability in polymer science,’ comments Marc Hillmyer, Director of the Center for Sustainable Polymers at the University of Minnesota, US.
In the future Nicholas and his team hope to better understand the mechanism of the catalysis, so as to be able to expand the substrate scope, and be able to depolymerise more plastics more efficiently and cheaply.
References
- E M Krall et al, Chem. Commun., 2014, DOI: 10.1039/c4cc00541d (This paper is free to access until 2 May 2014)
- J Zhang et al, J. Am. Chem. Soc., 2005, 127, 10840 (DOI: 10.1021/ja052862b)











No comments yet