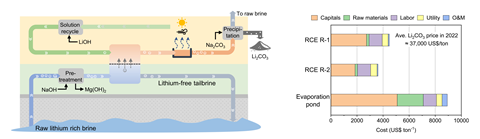
A new electrodialysis technique that directly and continuously extracts lithium from brines could be around 40% cheaper and have a much smaller environmental footprint than the most common mining method. Currently, the metal is mainly mined by pumping natural lithium brines from underground aquifers into evaporation ponds and this takes a great deal of energy and chemical processing, as well as vast areas of land.

If the method can be scaled up, the work could help meet the ever-growing demand for the metal for electric vehicle batteries and renewable energy storage, while lowering its price, according to the researchers.
Electrodialysis has gained momentum in recent years as a potential lithium extraction method due to the availability of highly selective separation membranes. The concept essentially uses an electric field to selectively sieve lithium ions in solution – which could be brine or seawater – by forcing them through a semi-permeable membrane to produce more concentrated solutions from which lithium can be extracted.
However, while recent prototype systems using state-of-the-art solid-state electrolyte (SSE) membranes have been developed, they have remained energy intensive – due to the high voltages they require – and inefficient, thereby undermining their economic viability.
Now, Yi Cui and his colleagues at Stanford University, US, believe they have overcome these challenges by developing a new electrodialysis approach – dubbed redox-couple electrodialysis (RCE) – for continuous lithium extraction that, crucially, reverses the half-redox, or water-splitting, reaction that is the usual driving force for electrodialysis.
‘The benefits to efficiency and cost in our approach make it a promising alternative to current extraction techniques and a potential game changer for the lithium supply chain,’ says Cui. ‘Eventually, we hope our method will significantly advance electrified transportation and the ability to store renewable energy.’
The team created a set-up with left and right chambers containing the cathode and anode, respectively, separated by a commercial SSE membrane. A lithium hydroxide solution was then fed into the right chamber, where the anode reduced water, producing hydroxide and hydrogen gas via the hydrogen evolution reaction – the half-reaction of electrochemical water splitting. In normal electrodialysis, the hydrogen evolution reaction is usually coupled with the oxygen evolution reaction at the cathode. However, in the team’s set-up, the hydrogen from the half reaction was fed into the left chamber that was also supplied with a lithium-rich brine. The hydrogen then reacted with the hydroxide in the brine to form water at the cathode.
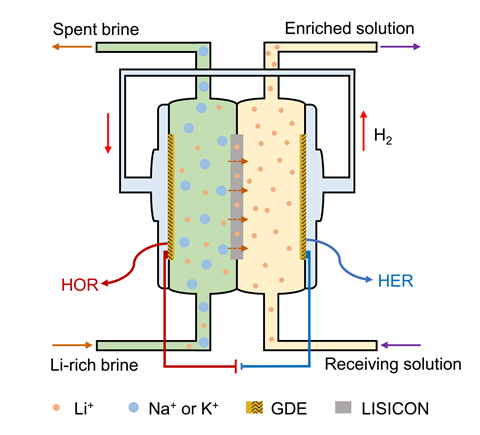
This redox-couple reaction led to the build-up of an electric charge within the electrolyte, driving lithium ions to migrate from the left chamber to the right, enriching the lithium hydroxide solution. However, as the redox-couple reaction was reversed to oxidise hydrogen instead of producing oxygen, the team discovered that the extraction process worked at an ultra-low voltage of around 100 millivolts. By comparison, other recent prototype electrodialysis systems have needed 3V or more.
The method worked with a variety of saline waters, including those with varying concentrations of lithium, sodium and potassium, as well as with wastewater from oil production. The researchers also quadrupled the size of their lab system, demonstrating that the RCE method remained energy efficient and highly selective. ‘This suggests that the method could be applied on an industrial scale, making it a viable alternative to current extraction technologies,’ says Cui.
‘The key innovation here is the so-called redox-couple electrodialysis – the performance is very good in terms of high selectivity and low energy consumption,’ comments Shihong Lin, a resource extraction and separation expert at Vanderbilt University, US. ‘However, the problem for real applications is that the commercial SSE [membrane used] is very expensive, thus scaling up is very challenging.’
Cui, however, remains optimistic. ‘There has not been large commercial use of this type of membrane, so its selling price at the moment is high but there is no reason why this cost cannot be reduced by many orders of magnitude,’ he says. The team are now looking to improve membrane stability and increase the extraction rate, which includes investigating alternative membrane materials.
References
R Xu et al, Matter, 2024, DOI: 10.1016/j.matt.2024.07.014












No comments yet