What makes an ionic liquid viscous?
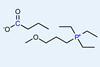
Elegant experimental work reveals that the runniness of ionic liquids is governed by a delicate balance between two phenomena
Experimental work from an international team of scientists has determined the relative contributions from two competing theories that seek to account for why an ionic liquid is viscous.
