An artificial biosystem comprising three microbes and a conductive polymer has been shown to produce proteins using sunlight, atmospheric carbon dioxide and nitrogen more efficiently than natural organisms. The multi-organism approach could enable symbiotic microfactories that synthesise commercial biochemicals useful for agricultural, environmental, food and medical applications, say the researchers.
Individual microbe species have been used to produce natural products for decades. However, systems that artificially combine the different abilities of microorganisms to work together symbiotically could open up more environmentally-friendly and efficient production routes. The problem is that symbiotic relationships have limitations because the communication of electrons and chemicals between microbes is inefficient, resulting in low yields.
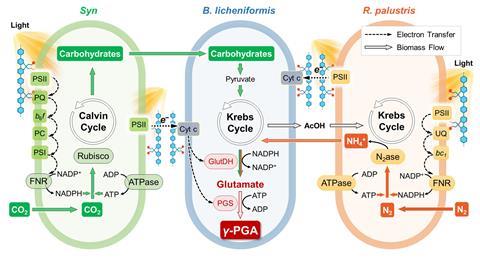
Now Haotian Bai and his colleagues at the Chinese National Academy of Sciences in Beijing have developed a proof-of-concept photosynthetic artificial symbiont in which three microbial components work together to make polypeptides using sunlight, carbon dioxide and nitrogen. Crucially, conducting polymers in the system overcome previous efficiency limitations by enhancing the direct transfer of molecules and electrons between species.
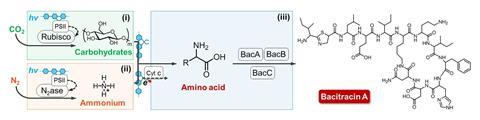
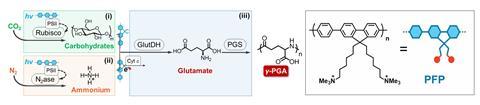
The team set out to design a system that could produce two target proteins that have naturally low biosynthesis efficiencies. The first, γ-polyglutamic acid (γ-PGA), is used in the food industry, pharmaceuticals and water treatment. The second, bacitracin A is an antibiotic. ‘Our approach involved careful selection and testing of different organisms to determine which combinations would work best together in our biosynthesis system,’ explains Bai.
The team started with the soil-dwelling bacterium Bacillus licheniformis, which has metabolic pathways for producing γ-PGA and bacitracin A. However, alone it is unable to synthesise them from carbon dioxide and nitrogen using solar energy. To that end, the team identified a freshwater cyanobacteria belonging to the Synechocystis genus as an ideal partner that could fix carbon via photosynthesis and supply the system with a necessary source of carbohydrates. Meanwhile, a nitrogen-fixing bacterium Rhodopseudomonas palustris was employed to convert atmospheric nitrogen into ammonium, which is needed as a feedstock by Bacillus licheniformis to produce the target proteins.
‘A combination of the three organisms can produce polypeptides but is inefficient,’ says Bai. To boost the efficiency, the team then introduced a conducting polymer made from poly(fluorene-co-phenylene). ‘We believe that the conductive polymer enables the multi-biological symbionts to form a network and achieve stable transfer of molecules and electrons,’ explains Bai.
Results showed that the yield of γ-PGA increased by 104%, and the photosynthetic efficiency doubled from 0.71% to 1.43%, compared with tests without the conducting polymer. When the system was designed to produce bacitracin A, the yield increased by 77%. ‘This low-cost method for producing high-value products has significant implications for sustainable manufacturing,’ says Bai.
However, Patrik Jones who studies synthetic metabolic pathways in microbes at Imperial College London, is unconvinced. He points out that many of the observations in the study concerning molecular transport, particularly of ammonium, have incomplete cause and effect relationships and some of the key interactions remain unverified. ‘It is interesting in terms of multi-species complexity and interactions with non-biological process elements, but it is unlikely to be commercially competitive and would likely be difficult to replicate [on a commercial scale],’ he says.
References
W Yu et al, Sci. Adv., 2023, DOI: 10.1126/sciadv.adf6772








No comments yet