A technique full of potential
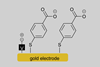
Using voltage to fine-tune reactivity
When synthetic reactions are discovered, organic chemists typically report a set of conditions that works for a large range of substrates. We understand that this is in fact a compromise as the optimal conditions for any individual substrate may vary significantly.
The multitude of reasons for this difference of reactivity between substrates are often lumped together as ‘stereoelectronic effects’. However, the inductive effect on reactivity – electron-withdrawing or donating through sigma bonds of nearby functional groups – reported by Louis Plack Hammett in 1937 is perhaps the most fundamental and also conceptually intuitive process underlying our understanding of chemical reactivity. In its simplest form: opposites attract.