There are many ways of discovering truth. The scientific method is one of the best. It depends on the carrying out of repeatable experiments. Science cannot really deal with a unique event. So I am surprised that Philip Ball (Chemistry World, April 2011, p33) dismisses so easily the practicality of replicating experiments. However, Ball rightly recognises the near impossibility of exactly duplicating the experimental conditions of others’ work. As chemical researchers, surely we all repeat our own experiments many times over before publishing the results.
I published a paper in Chemical Communications about the electrochemical oxidation of hydroquinone. Soon afterwards a person in Sweden published a paper criticising my work and showing different results (also in Chem. Commun.). I quickly realised that he had used lithium perchlorate as the supporting electrolyte, whereas I had used tetraethylammonium perchlorate. The lithium ion had acted as a Lewis acid causing a proton donor effect. So I repeated his work and my own again and published another paper in Chem. Commun. explaining the discrepancy. That is how science should work.
Many truths are described in anecdotal observations by credible witnesses. But do we not do the same? We describe what we observe (or think we observe) in our experiments and then publish them. How much more reliable is our truth than that of witnesses to a unique event, such as a crime or a motor accident?
B R Eggins, FRSC
Ulster, Northern Ireland
In his recent letter to the magazine, Robert Slinn writes ’At the moment, we desperately need a chemist at the forefront advising this coalition government....’ (Chemistry World, April 2011, p37).
Without trying to be too political, many of us can remember the last time we had a chemist in high political position. That didn’t turn out too well for UK manufacturing as a whole.
Is the reverse true? Perhaps with this present cut-based government, if we have a little less profile then we might be left alone politically and less likely to attract cuts?
I do agree with Robert’s comment about emphasis on ’in-house work-based training’ or apprenticeships. These need to be formalised so that employees can provide evidence to future prospective employers (a similar concept to CPD [continuing professional development] ).
D Garratt MRSC
By email
With reference to David Jones’s Last Retort article (Chemistry World, April 2011, p72), I recall reading in a textbook (Hildebrand & Latimer) in about 1950 a description of a six component immiscible system [hexane, aniline, water, white phosphorus (melting point 44°C), gallium (mp 30°C) and mercury]. Clearly this requires a temperature above 45°C.
I suspect that a fluorocarbon such as perfluorodecalin would form a seventh layer. Additionally, it is possible to get two immiscible aqueous layers (concentrated ammonia and saturated potassium carbonate solutions) although it may not be possible to combine these with the six or seven layer system at the temperature this requires.
G A Taylor CChem FRSC
By email
Layering miscible liquids is a must-have skill for bartenders and mixologists. I once watched my friend Giles layer 11 separate beverages one on top of the other in a glass in the space of less than five minutes. It was a spectacular feat. And the result was universally agreed to taste disgusting.
A Sella FRSC
London, UK
Andy Extance’s very interesting article on CO2 as a reagent (Chemistry World , February 2010, p40) prompts me to draw your attention to a related matter which may provide you with some unexpected subjects. In a major research paper on the polymerisation of isobutene (see reference, below), my co-workers and I reported also on some polymerisations which were done in liquid CO2 as solvent, necessarily at below -50°C and a pressure above 5atm; and we did it in a glass reactor!
I then committed the tactical error of not referring to that achievement or following it up with further publications. Thus this undoubted British ’first’ has been, if not forgotten, then certainly not widely recognised. Martyn Poliakoff (interviewed in the same issue, p31), who became a specialist in that area, certainly acknowledged our priority properly, but I did not follow up that venture with further publications on CO2 as solvent. Our experiments had been done in 1956 and in the summer of that year, as a guest-worker in the laboratories of the Polymer Corporation of Canada at Sarnia, Ontario, we did many more polymerisations in CO2 as solvent, but in steel vessels under proper industrial conditions.
The then research director, Bill Buckler, and I hoped that our pioneering work might lead to the replacement of methyl chloride (chloromethane) then used in the manufacture of polyisobutene and - much more important - of butyl rubber, with the virtually cost-free and harmless CO2. However, MeCl was so cheap and its harmful effects then unknown (so that leaks were unimportant), that no one felt it worthwhile to follow up our innovation, or take out a patent.
Peter Plesch CChem FRSC
Northampton, UK
[The following letter relates to a Classic kit piece on the ’Pardy apparatus’ slated for 1 April ( Chemistry World, April 2011, p60)]

I feel peculiarly honoured to have received recognition for this piece of equipment, when I never sought anything at all. As Ralph Waldo Emerson once wrote, ’the louder he talked of his honour, the faster we counted our spoons’.
Around 1976, I needed to distil larger quantities of some rather involatile and thermally unstable liquids, such as penta-alkyl-cyclopentadienyl-tributyl-tin. I simply adapted an older idea I’d learned from John Segal which used a cold finger condenser with a receptacle on the end. I never published the side arm device and it’s entirely possible that others independently had the idea long before me. It was only in 2004 that I discovered that people at Oxford had made my name a verb . and a noun. More complete details of the equipment I used can be found in the online version of this letter.
I was not consulted before publication. Needless to say, Andrea Sella’s article does contain a few biographical inaccuracies. Well, scarcely any truths in fact. My wife Elma particularly liked the bit about my being a naval commando (see below for further details).
Anyone who can find my pub in the village of Drax is welcome to a free pint. Just ask for the Cock and Bull and have a long weight with you.
R B A Pardy FRSC CChem
By email
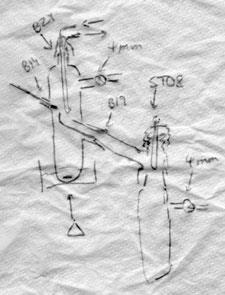
The late Arthur Finch and I, when assistant lecturers at Royal Holloway College in 1958-9, required an apparatus for vacuum distillation of boron compounds which were air-sensitive and which boiled at under 100°C in vacuo. Arthur (a skilled glassblower and vacuum line user) and I (then a conventionally trained organic chemist) came up with a short path distillation apparatus, using a cold finger as condenser, which could be attached to a vacuum pump, or into a vac line as required. These were essentially the equipment described in the April Fool spoof in your last issue (which certainly caught me). Arthur made these to any size as required.
I took several to the inorganic chemistry department at Newcastle University when I went there in 1962, and they were adopted and adapted by the Greenwood group who used vacuum line techniques for their air-sensitive materials. I retired from Newcastle some years ago but I suspect there are still some examples around the labs (if not already cannibalised for their ground glass joints). We never regarded these as particularly original - vacuum apparatus was always made or adapted for the particular physical properties of the molecules of interest. Others undoubtedly had similar ideas and still adapt apparatus for their own use.
J C Lockhart MRSC
Edinburgh, UK
I have noted a possible difficulty with the arguments in Ronald Dell’s recent letter (Chemistry World, April 2011, p36). In discussing heat balance at the ’surface’ of a human occupant of a room, Dell states:
’ . . . radiation is proportional to (T14 - To4) where T1 and To are the absolute temperature of the body and the surroundings’
To is in fact the temperature of the nearest absorptive surface, which in the situation under consideration will be the walls of the room. It is not the temperature of the air, the immediate ’surroundings’ of the occupant of the room, as this is transparent to thermal radiation. How close the temperature of the air is to that of the walls would need to be ascertained if this point was being examined experimentally. Further discussion of this point is to be found in my 2003 text Hydrocarbon process safety: a text for students and professionals (Whittles Publishing, Caithness, UK). There I make a clear distinction between ’the surrounding air’ and ’the surroundings’ for radiation heat transfer analysis and emphasise that in storage of hazardous chemicals there is no justification for assuming that temperatures of the two are equal.
Much more could be said and I shall be pleased to respond to enquiries on this matter, but I doubt whether the editorial staff of CW will be wanting to publish anything longer than this.
J C Jones FRSC
Aberdeen, UK
With regard to your article on the first purely organic phosphor (Chemistry World, April 2011, p30), I reported a phosphorescent organic compound in 1946. The substance concerned was N -phenylthiamorpholine-p -toluene-sulfonyl imine, formed by the reaction of chloramine T with N -phenylthiamorpholine, itself a product of the reaction of aniline with 2,2’-dichlorodiethyl sulfide.
On irradiation with ultra-violet light this substance exhibited a blue fluorescence and a bright green phosphorescence, having a half-life of a few seconds. I observed at the time that the phenomenon was unusual - I did not realise how unusual!
Unfortunately the work was reported in a classified PhD thesis, and was never published in the open literature. I hope that it may now be of some interest.
A F Childs CChem FRSC
Kinver, UK
The recent April Fool in Chemistry World1 about some equipment I devised about 35 years ago made me feel peculiarly honoured. I never published it, yet two or three publications mention it,2 so, since people are curious about it, perhaps now would be as good a time as any to record it.
The equipment is shown in Figure 1.
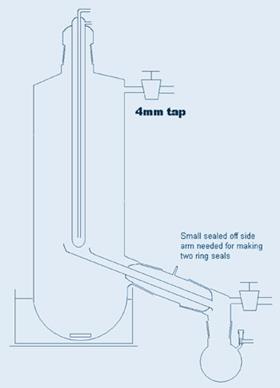
The key component in using this equipment is the magnetic flea, because as it revolves, it acts as an initiator for boiling and for some reason, droplets of liquid are thrown out of the vortex it creates. They land only on the sides of the tube, not on the central condenser. With care, one can make a device with only three centimetres or so between the surface of the boiling liquid and the cold surface of the condenser. The end of the receiver can be attached to a conventional receiver adapter as shown or a distillation pig or indeed a suitably connected Schlenk tube. I’ve shown a slightly more sophisticated side arm receiver which minimises mechanical losses. Side arm round bottom flasks would be a good choice of receptacle in my opinion, because they’re more compact.3 I didn’t bother with a thermometer pocket - one can measure the oil bath temperature to ensure it doesn’t get too hot. If the oil bath or heater surrounding the Schlenk tube covers most of the distance to the side arm, the vapour has hardly any distance to travel and the large cross-section makes it possible to create a hard vacuum, minimising the temperature and with plenty of space for the volume of vapour. The side arm joint must be close to the side of the Schlenk tube to avoid distillate collecting in that space. Sometimes that joint may need a little freshening up with fine carborundum after the equipment is made, although a good glassblower should be able to manage it without scorching the joint.
Richard B A Pardy FRSC CChem
Notes
- Andrea Sella, Chemistry World, April 2011, p60, I had no involvement in the writing of the article.
- The diagram of a Schlenk modified with cold finger carrying a receptacle is in my thesis, but not the side arm thing which members of the Green group have so kindly associated with my name, even though there may be many prior claimants.
- A Young’s tap is preferable to Rotaflow especially if you want to put the product in the fridge afterwards, because the Teflon shrinks and will let in air and moisture. They’ve probably improved the design since I used them - it’s been twenty years after all.
The recent April Fool in Chemistry World contains completely false biographical data about me which I wish to correct.
Please see a recent picture of me - I never wore a beard of any kind.

Born in the Black Country to the West of Birmingham in the early fifties, my father was a Bristolian, an electrical engineer, who worked for the MEB. My brother is a few years older than I am. When I was eleven we moved out of suburbia into the Worcestershire countryside. My surname comes from Bristol and the spelling of my ancestors’ surname changed from Pardey in the 1850s, but dates back many centuries and is linked to many other names with the same stem. My brother and I attended a small and ancient local grammar school which of course no longer exists as it was, and I wish to record my most sincere thanks to the teachers there who gave me so much encouragement. Right from the start, I was particularly keen on chemistry, and used to do experiments in the garden shed. This had become necessary after I produced a flame of about two feet high from a Thermite reaction in my bedroom. It rather startled my mother because the curtains were only about a foot away, though personally it all seemed perfectly fine (at the time at least). Later on I used to make Grignard reagents and even tin alkyls at home, having made my own tin tetrachloride and the chlorine for it by traditional means. They weren’t pure but the smell was. unmistakeable. I left school at eighteen with a socially unacceptable number of GCEs and at York University, I received a technical grounding which I’ve never found to be deficient.
Having graduated with a first from York in 1974, I was very fortunate to be accepted as a student by Malcolm Green at Oxford and to be admitted to Balliol College as a graduate student. Sella’s rumour I was a Navy commando while being very appealing is groundless. I was always absolutely hopeless at sport. I did get an RYA Day Skippers’ certificate later on, but it’s not the same thing. at all.
After Oxford, I joined Prof. E.O. Fischer’s group in Munich for eighteen months, being funded by a Royal Society Science Exchange Fellowship. I made a tin substituted chromium carbene complex of which Ulli Schubert miraculously did an X-ray structure, but I don’t think anyone has shown the least interest in it since then. The next nine months were with Prof. Igor Tkatchenko in the CNRS in Lyons, working on some nickel catalysts and then two years on an SERC Fellowship at Cambridge with Jack Lewis. I continued some studies on the cobalt systems I’d made at Oxford, finding first a model reaction for the Cossee mechanism and then synthesising a highly reactive Cp*cobaltbis(ethylene)hydride cation which was both fluxional and had the prototypical agostic C-H bond. I remain extremely grateful for the opportunities these fellowships gave me.
During this time I’d hoped to gain an academic position, but it became apparent that the funding simply wasn’t there, and the politics had become completely hopeless. It seemed wrong to keep pestering my ex-supervisors for references. I made a number of applications and accepted a job with BP Chemicals in 1981. After two years at Grangemouth, the group I was in was transferred to Hull. I went with my new wife, Elma, who had been the division manager’s secretary (this came as a complete surprise to the division manager). We have a son and now also a grandson, I’m very happy to say. During that time, I took a Diploma in Management Studies at the local business school and would recommend chemists to consider such a course. For a few years I wrote various kinds of technical reports. This took me into the processes of finding and providing information. I wrote a monthly summary of Chinese business news for five years as a side line but that’s as close as I ever came to China or anywhere else in the Far East. The last ten years were spent creating a document repository of about half a million documents - the heritage of the chemicals sides of both BP and Amoco. When Innovene (now businesses in Ineos) was divested, that repository helped BP to realise about $9billion for the sale of those businesses by allocating ownership.
My main interest has always been research. I never regretted going into industry, and however frustrating it has been, it would have been far worse in university. There are a few patents from my time with BP. One of them ought to get more attention than it does, but I doubt it ever will. I volunteered to retire in 2007.
Concerning my wife, she has been in only moderate health for several years, having suffered the consequences of two very major operations. I spend much of my time caring for her. We did find Sella’s article very funny - it gave her the best laugh she’s had for some time.
Richard B A Pardy FRSC CChem











No comments yet