Dylan Stiles just loves those funny-looking molecules
The rise of nanotechnology has brought a resurgence of interest in one of my favorite molecules, buckminsterfullerene. First discovered in 1985, it’s one of the more recent additions to a family I like to call the ’funny-looking molecules’.
Their preparation has a rich history that is almost as old as organic chemistry itself. Why do chemists bother to synthesize these things? In many respects the goal is to test the physical limits of organic molecules. Left to its own devices, an sp3 C-C bond is most comfortable with a bond distance of 1.54 Å and angle of 109.5°. But how far can we stretch that before it breaks? Synthesizing molecules that force atoms into bizarre contortionist acts is the only way to learn.
I’ve assembled a small collection of the world-record holders below, categorizing them as things that have or have not (yet) been prepared. Note the distinction between ’prepared’ and ’isolated’ - cyclobutadiene will spontaneously dimerize at room temperature, but trapped in an argon matrix it has nowhere to go.
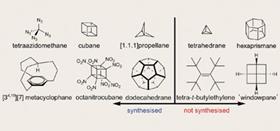
Some of the molecules that have been prepared seem to defy logic. In [1.1.1]propellane, four C-C bonds point into the same hemisphere, like a tetrahedron turned inside out. Just try to build a plastic version of this molecule without breaking your model kit. It takes some serious head-scratching to explain why it’s relatively stable. The fact of the matter is that [1.1.1]propellane would rather be just about anything else, but it has no energetically feasible way of getting there. Tricking molecules into a local energetic minimum is a recurring theme in this field.
The so-called Platonic hydrocarbons deserve special mention. Only the tetrahedron, cube, and dodecahedron have conceivable hydrocarbon analogues, and while cubane and dodecahedrane have been prepared, tetrahedrane seems too unstable to exist - a hydrocarbon unicorn.
Paquette’s conquest of dodecahedrane, the Mount Everest of molecules, was a remarkable achievement. Most organic syntheses build molecules from the bottom up. Functional groups are introduced, molecules become larger, and 13C NMR spectra grow a forest of peaks. The synthesis of dodecahedrane, every bit as lengthy as many natural products, is almost the reverse. Polar groups in the starting materials are used as synthetic handles and successively discarded through reduction and photoextrusion. As the symmetry of the molecule increases, the NMR spectra become simpler and simpler until the product is reached. It has only one peak in both the carbon and proton NMR.
Many funny-looking molecules still evade synthesis, however. Tetra-tert-butylethylene probably takes the cake in this category. Numerous fully-substituted alkenes have been made, but an alkene bearing four tert-butyl groups is yet to be prepared, and it’s not for want of trying. Stubborn chemists keep chasing this beast because it has been predicted to be stable.
All the molecules mentioned so far are constructed only from carbon and hydrogen. What happens when you take a highly-strained core and start attaching heteroatoms? Molecules like octanitrocubane are mostly interesting as high explosives, as they pack the most bang for your buck. It’s a risky business, and the paper describing the synthesis of tetraazidomethane is accompanied by a menacing safety disclaimer and tales of exploded lab equipment.
Of course, I’ve only scratched the surface here. To delve more deeply, allow me to wholeheartedly recommend Henning Hopf’s Classics in Hydrocarbon Chemistry (Wiley-VCH, 2000). Flipping through the pages of this book is a stroll through an art gallery of triangles and hexagons attached in manners most unusual.
Perhaps the most appealing aspect of these molecules is that synthesizing them can be the purest form of ’playing with atoms’. All you have to do is start doodling with a pen and paper, imagine a structure that pushes atoms to their limits, and question whether it can be made. Odds are some chemist is likely to take your wager.












No comments yet