Reflections on the isolation of radicals

For chemists, the word radical has changed in strange ways. Derived from the Latin radix, root, the word referred originally to stable groupings of atoms that stayed together during chemical reactions. The R groups of today are direct descendants of the radicals of yesteryear, when organic chemists built homologous series and identified compounds by disconnection and functional group transformation.
But the Rs themselves were elusive. Joseph-Louis Gay-Lussac tried to isolate CN, but obtained cyanogen, (CN)2. Robert Bunsen too, failed to isolate ‘free cacodyl’ (AsMe2), getting its dimer instead. His student Hermann Kolbe noisily claimed to have isolated ‘free methyl’ by electrolysis. He hadn’t.
Another Bunsen student, Edward Frankland, tried to isolate ’ethyl’ using zinc, in epic, fiery experiments that yielded, among other things, butane. It was not until 1900 that the first properly observable radical (in the modern sense), triphenylmethyl, was isolated by Moses Gomberg at the University of Michigan, US.
The question left hanging in the chemical air was whether a methyl could have an independent existence. This caught the eye of Friedrich Paneth, a remarkable chemist’s chemist.
Born into an intellectual Viennese family, Paneth became a highly skilled experimenter – he would be remembered for his delicacy of touch in the lab. He got his doctorate in 1910 with Zdenko Skraup, a natural products chemist famous for working out the structure of quinine by classical analysis, and making the initial steps towards its synthesis. Paneth’s thesis explored the reactions of quinidine and of proteins, but bio-organic chemistry must have lost its appeal.
Tracing a new path
In 1912, Paneth became assistant to Stefan Meyer, the boss of Vienna’s Institute for Radium Research. Marie Curie had just won her second Nobel prize for her isolation of radium and polonium. This was much more exciting. His work with Meyer involved unravelling the radioactive decay chains of uranium and its daughter products. The work took place on minute scales, and would have brought Paneth in contact with the microchemical work of Friedrich Emich and Fritz Pregl. This led him to analytical methods using radioactive tracers and dyes.
By 1929, Paneth had been appointed chair of chemistry in Königsberg (then in Germany, but today the Russian exclave of Kaliningrad). His work on radioactive heavy elements led him to studies of hydrides, hoping that their volatility might make them useful tools for tracer experiments. After making bismuth, tin and even polonium hydrides, he and his students got nowhere with lead until they used lead electrodes to generate sparks in a flow of hydrogen gas. A delicate mirror appeared a little further along the tube, much as James Marsh had seen a century earlier with arsine.
The extreme instability of this species led Paneth to explore the decomposition of lead tetramethyl – a somewhat more stable, but highly volatile compound – which gave clean mirrors when heated in a quartz tube using a Bunsen burner. In a stream of hydrogen, a mirror deposited at one point in such a tube could later be erased if a second mirror was deposited further upstream. This meant that the elusive free methyls were being formed thermally and then reacted with the second mirror to reform the original compound.
Purity was critical to the experiments. The carrier gases hydrogen and nitrogen, made chemically, were meticulously scrubbed. With the vapour pressure of lead tetramethyl fixed using a dry ice/acetone bath, reproducible mirrors could be made along the tube by heating the flowing gas. A downstream mirror could now be used analytically to report on the radicals produced in a hot zone further upstream. The time required to erase a mirror of known mass gave the concentration of gas phase radicals; it varied as a function of the distance between the cold mirror and the hot zone.
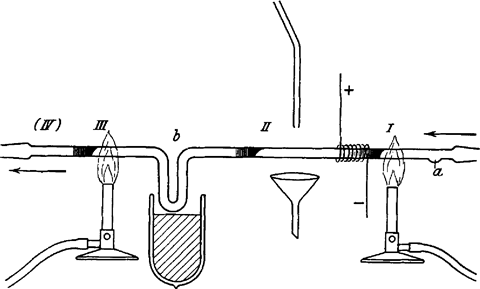
These extremely simple observations led to kinetic plots from which the lifetime of methyl radicals could be inferred. The methyl radicals themselves could be reacted with other metal mirrors to make further organometallics, each identified by microchemical methods.
This elegant chemistry ended in 1933. While Paneth was on a lecture tour of the UK, Adolf Hitler became chancellor of Germany. Although brought up a Protestant, Paneth’s parents were Jews; he read the writing on the wall and decided not to return home. He got a fellowship to work at Imperial College in London and was then appointed professor at Durham University. He returned to radiochemistry, determining the age of meteorites by measuring minute amounts of helium produced by radioactive decay. He even shot down the suggestion that helium was produced from hydrogen absorbed by palladium, some 50 years before the cold fusion brouhaha.
Paneth’s studies put radicals on the chemical map – without any spectroscopy – laying the groundwork for the study of combustion and photochemical reactions that would be conducted by other means. With the word radical on the lips of certain politicians these days, could Paneth’s thoughtful prescience about politics prove to be an object lesson for us?
References
F Paneth and W Hofeditz, Ber. dtsch chem. Ges., 1929, 62, 1335 (DOI: 10.1002/cber.19290620537)

















No comments yet