Thapsigargin
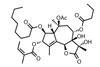
A complex synthesis is always more impressive when conducted on gram scale
Why do we do total synthesis? Regular readers of this column will have seen plenty of the exciting new methods that chemists can discover when forced to innovate to reach a new target. And even in this age of gigahertz NMR, benchtop density functional theory calculations and high resolution x-ray crystallography, total synthesis plays a role in structure elucidation. More than anything, I hope I’ve been able to provide a glimpse of nature’s molecular beauty, and how total synthesis is an edifying intellectual (and even artistic) pursuit.