Bedaquiline
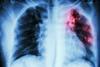
The first TB drug approved in 40 years gave hope to sufferers of drug resistant tuberculosis and ushered in a new class of antibiotics
The first TB drug approved in 40 years gave hope to sufferers of drug resistant tuberculosis and ushered in a new class of antibiotics