Ben Valsler
Pull out your quill and inkwell and prepare to take notes – Mike Freemantle has a test for you…
Michael Freemantle
Here’s a quiz question for you. What links the Italian Renaissance artist Leonardo da Vinci, 18th century German composer Johann Sebastian Bach, oak trees and ferrous sulfate?

Did you get it?
The link is ink. Specifically, a black ink known as iron gall ink.
Da Vinci used iron gall ink for his drawings and to write his journals. Bach used the ink to write his musical scores.


The ink was well known in Roman times and widely used throughout Europe for centuries, especially during the Middle Ages. It was typically prepared by first boiling oak tree galls in water to leach out tannins. Tree galls are the external localised swellings that grow on trees, usually in response to attacks by pests or parasites. You might know them as ‘oak apples.’

Oak gall tannins are particularly rich in tannic acid, a polyphenolic compound also known as gallotannic acid. The galls also contain smaller amounts of gallic acid, a phenolic compound with three hydroxyl groups.
The iron compound used to prepare iron gall ink was ferrous sulfate – also called copperas or green vitriol, more commonly known nowadays as iron(II) sulfate. It is made by adding sulfuric acid to iron, or by oxidising pyrite, a widespread and abundant iron sulfide mineral. It is one of two iron sulfates. The other being ferric sulfate or iron(III) sulfate.

Addition of ferrous sulfate to oak gall extracts produced a greyish solution of iron(II) tannate and iron(II) gallate complexes. When applied to paper or parchment, the solution penetrated the surface, and then, on exposure to air, the iron(II) complexes oxidised to form black iron(III) complexes that are insoluble in water.
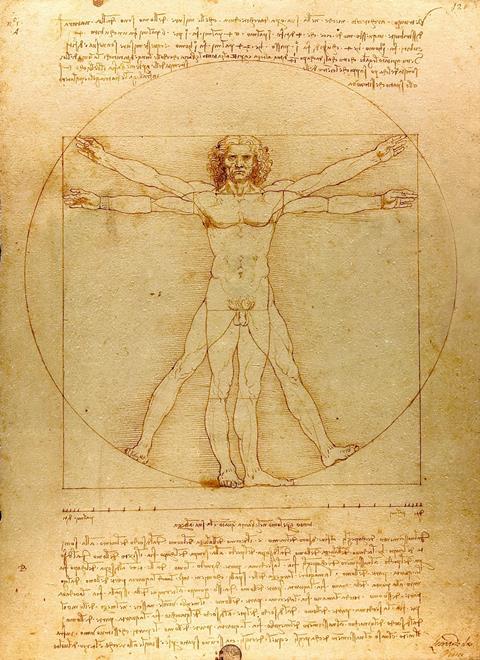
Numerous recipes were used during the Middle Ages to make the ink. Some ink makers allowed the oak gall extract to ferment for several days. This increased the amount of gallic acid in the solution and produced a darker ink. During the fermentation process, tannase, an enzyme that occurs naturally in oak galls, catalysed the hydrolysis of gallotannic acid to gallic acid. The same result could also be achieved by adding an acid, such as vinegar, to the solution.
Gum arabic was sometimes used to improve the quality of the ink. This naturally-occurring honey-coloured resin is extracted from acacia trees native to Africa and the Middle East. The gum thickened the ink and improved its surface tension on the pen nib. This enabled more ink to be carried on the nib after each dip into an ink well. It also acted as a binder to keep the pigment in suspension and prevented the ink from soaking into paper or parchment too quickly.

Before the advent of iron gall inks, the primary ink used for writing and drawing was carbon ink, also known as Indian or Chinese ink. Its use can be traced back to at least 2500 BC. Carbon inks were typically made by mixing lampblack, soot or some other form of carbon, with a binding agent such as animal glue. The mixtures were then stirred with water to create a suspension. But unlike iron gall inks, carbon inks tended to smudge and could be readily washed out of a document or drawing. Ink gall inks were considered superior because they were indelible, meaning that they could not easily be erased from paper or parchment.

Many historical iron gall inks, however, suffer from two major disadvantages, most notably when they were used on paper. Firstly, they tend to fade over time. Secondly, they can degrade the paper itself, leading to its eventual disintegration in a process known as ‘iron gall ink corrosion.’
The problems are caused by the acidic nature of the inks and the use of excess amounts of ferrous sulfate in some of the old recipes. The surplus iron(II) ions react with atmospheric oxygen to form iron(III) oxide which has a paler colour than the iron(III) organic complexes. The ions also catalyse a chain reaction that generates hydrogen peroxide, a bleaching agent, and hydroxyl radicals that attack the cellulose in the paper.

Many libraries, archives and museum collections throughout the world contain ancient paper manuscripts written in iron gall ink and artistic works drawn with the ink. Their preservation before they fade and completely deteriorate is an immense challenge that continues to attract the attention of conservation scientists.
Ben Valsler
That was Mike Freemantle with ferrous sulfate – the iron heart of oak gall ink. Next week, Kit Chapman explains how chemists can save the structure of a sunken shipwreck
Kit Chapman
If the Vasa was just left to dry, it would shrink, warp and collapse. Desperate to look after their wreck, the Swedish team decided to turn to chemistry.
Ben Valsler
Catch Kit next time. Until then our usual lines of communication are open – email chemistryworld@rsc.org or tweet @chemistryworld. I’m Ben Valsler, thanks for listening.
References
Music: Prelude from Prelude and Fugue in a minor, BWV 543, from Johann Sebastian Bach. Performed by Noah Horn. Released under a CC-BY 2.5 licence.











No comments yet