Phosphoric Acid
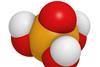
Lars Öhrström on phosphoric acid: Crucial for crops and DNA’s backbone, it’s the energy currency of the biological world
Lars Öhrström on phosphoric acid: Crucial for crops and DNA’s backbone, it’s the energy currency of the biological world