Quantum and theoretical chemistry – Page 10
-
 Research
ResearchQuantum computer simulates hydrogen molecule
Prototype shows potential for more complex electronic structure calculations
-
 Research
ResearchScientists finally calculate water’s freezing point from scratch
Machine learning shows how van der Waals forces help explain watery wonders like floating ice cubes
-
 Research
ResearchAll-metal sandwich inspires a theoretical following
Recently discovered [Sb3Au3Sb3]3– has multiple groups hunting for answers
-
 Research
ResearchIron-rich silicate plays cosmic matchmaker
Findings could help explain abundance of molecular hydrogen in interstellar space
-
 Research
ResearchScientists claim to have seen ‘new state’ of water
Scepticism that water molecules with delocalised protons can be described as an unknown phase of water
-
 Opinion
OpinionHoming pigeons should thank quantum chemistry
Philip Ball asks whether quantum biology holds the secret to how birds navigate
-
 Research
ResearchBonding accurately predicted in diatomics by new scheme
Hopes that simple theoretical framework can be extended to tackle polyatomic molecules
-
 Research
ResearchRewriting the textbooks with a pinch of salt
Chlorine atoms in the –2 oxidation state, and other high pressure-induced oddities, predicted using new model
-
 Research
ResearchGroup 6 diatomic bonding is all relative
Relativistic effects may help to explain break from periodicity
-
 Research
ResearchElectron work functions look tough
Surface property calculations provide a link between the electron work function and toughness of transition metals
-
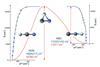 Research
ResearchGetting the measure of transition states
Previously impossible to monitor properties of transition states found hidden in vibration spectra
-
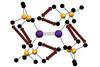 Research
ResearchSubtle forces yield profound effects on heavy element bonding
Calculations on dispersion forces shed light on interactions between heavier main group elements
-
 Research
ResearchHypervalent bonding controversy out for the electron count?
A new definition for the foundations of modern chemistry puts atomic charge models on trial
-
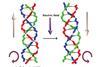 Research
ResearchElectric switch makes helix change hands
Computational model predicts that external electric fields can reorientate helices by breaking and reforming hydrogen bonds
-
 Research
ResearchSimple reaction shows quantum interference
Chemical reactions can show interference patterns similar to those seen in the classic double-slit experiment
-

-

-
 Opinion
OpinionDoes life play dice?
Philip Ball wonders whether life evolved to exploit quantum phenomena, or if it’s just in our nature
-

-
 Research
ResearchChemistry gets strange at water’s surface
Theoretical study suggests that ions with the same charge might actually become attracted to each other at an interface