In search of ultimate selectivity
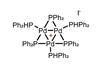
A catalyst that reacts only with aryl iodides, spurning bromides and chlorides
As funding for total synthesis has dwindled, the number of graduates combining extensive practical knowhow and outstanding retrosynthetic vision has declined, to the dismay of many. But does it really matter? Computational retrosynthesis programs that also select optimal reaction conditions are in the offing, and like it or not, computers will soon be good enough to replace a significant portion of human input in synthesis design. So what next for organic chemists? We are clearly not out of a job: great practical skills and chemical knowledge are still crucial to sense-checking and executing any synthesis a computer spits out, but these new tools give us the opportunity to redirect our energies.