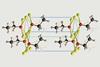
Sodium ethoxide crystal structure solved after 180 years
Analysis of reagent commonly used in Claisen condensations and malonic ester synthesis
Scientists in Germany have finally resolved the crystal structures of sodium ethoxide along with its corresponding ethanol disolvate.