The latent threat of tuberculosis
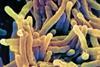
Although TB was close to being eradicated in the developed world, it is a major problem in developing countries. With drug-resistant strains on the increase, Clare Sansom outlines the latest in the fight against this killer disease