Nina Notman looks at the revolutionary treatment already taking on cancer, now aiming for wider use
In 2010, five-year-old Emily Whitehead was diagnosed with acute lymphoblastic leukaemia (ALL). Two years later, she had relapsed twice and exhausted all approved treatment options. Doctors were predicting she had just a few weeks to live. In an effort to extend her life, Emily’s parents enrolled their daughter in a Phase 1 clinical trial for a completely new type of therapeutic: CAR (chimeric antigen receptor)-T cell therapy.
The risks were extremely high. CAR-T therapies had only been tested on a handful of adults before and were known to cause cytokine storms, which are dangerous and sometimes fatal overreactions of the immune system. In April 2012, Emily became the first ever child to receive CAR-T cell therapy. She survived the treatment and her cancer rapidly went into complete remission. A decade later, Emily is still cancer free.
The CAR-T cell therapy that Emily received was Kymriah, developed by the immunotherapy pioneer Carl June and his team at the University of Pennsylvania, US, and licensed to Novartis. In 2017, Kymriah became the first gene therapy approved by the US Food and Drug Administration (FDA). It was first authorised as a drug-of-last-resort for treating ALL and then for treating another blood cancer, diffuse large B-cell lymphoma. Kymriah is now used as against both indications in many countries, including the UK.
Once in the body, CAR-T cells seek out and destroy malignant cells
Kymriah’s approval opened the floodgates for CAR-T cell therapies. Four others are now authorised for treating a variety of blood cancers – Yescarta and Tecartus marketed by Kite Pharma and Bristol-Myers Squibb’s Breyanzi and Abecma. Many other CAR-T cell therapies are in the pipeline with more than 600 clinical trials currently underway. Most are aimed at treating blood cancers, but CAR-T cell therapies are also being developed for solid cancers, HIV, autoimmune diseases and even heart attacks. Researchers are also developing cheaper and easier ways to deliver CAR-T cell therapies to those in need.
A game changer
T cells are white blood cells with a vital role in our immune system. They host proteins called T cell receptors on their surface, and as the T cells circulate the body, these receptors recognise antigens on abnormal or infected cells. Then, depending on the type of T cells they are, they either destroy the damaged cells themselves or stimulate the other white blood cells around them to do so.
A chimeric antigen receptor is a genetically modified version of the T cell receptor that recognises specific antigens on a particular type of diseased cell. Most of the approved CAR-T cell therapies to date target CD19, a protein that is commonly found on the surface of malignant B cells. B cells are white blood cells that – when healthy – produce antibodies to help the body fight infection
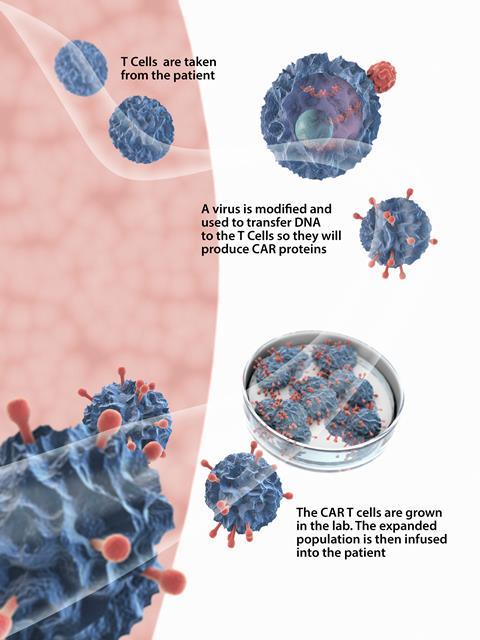
The manufacturing process for all the approved therapies is the same. First, T cells are harvested from a patient’s blood by passing it through a machine that separates blood into its various components. The rest of the blood is then returned to the patient. The T cells are then sent to a centralised manufacturing facility where their genetic material is modified. A lentiviral or retroviral vector delivers the new genetic information to the T cell by attaching itself to the cell surface and injecting RNA into its cytoplasm. This genetic information is essentially the recipe needed to produce the CAR. The genetic recipe is then incorporated into the T cell’s genome, so the protein it codes for (the CAR) is synthesised and expressed on the surface of the T cell. The CAR-T cells are then multiplied, frozen and returned to the hospital for infusion back into the patient. The process typically takes several weeks.
Once in the body, the CAR-T cells seek out and destroy malignant cells that have expressed the target antigen on their surface. They should then multiply and remain circulating around the body indefinitely. This means any new cancer cells expressing CD19 that appear later will be promptly destroyed. ‘They have the potential to be one-time curative therapies,’ explains Boro Dropulić, co-founder of Caring Cross, a not-for-profit that is developing treatments for cancers, HIV and other diseases.
A solid future
The basic design of the approved CAR-T cell therapies are pretty much the same as the first CARs, made in the late 1980s. There are, however, plenty of next generation CAR-T cell therapies in the pipeline with various design tweaks. Some of these engineered T cells target different antigens found on cancer cells’ surfaces and others have more than one antigen target. Some also have additional functionalities that boost potency and longevity. These tweaks should improve treatment success rates and expand the range of blood cancers CAR-T cell therapies can be used for.
CAR-T cell therapies are also in development with CARs designed to target solid tumours. ‘More than 90% of cancers are solid tumours and traditionally solid tumours are more difficult to treat than blood cancers,’ explains John Maher, head of the CAR mechanics group at King’s College London, UK.
It’s a challenge to design T cells to go and seek out those malignant tumour deposits
Blood cancers are the low-hanging fruit for CAR-T cell therapies, with safer targets upon which the technology has cut its teeth. ‘When you treat someone with a blood cancer with CAR-T, you put the cells into the bloodstream where they have direct access to the malignant cells. And even if the malignant cells are not in the blood but are in the bone marrow or the lymph nodes, these are still places that T cells like to migrate to,’ says Maher.
Solid tumours, by comparison, tend to hide away in locations such as the breast, lung, prostate and liver. ‘It’s a challenge to [design] T cells to go and seek out those malignant tumour deposits,’ explains Maher. And once they do arrive, the microenvironment they encounter is extremely hostile. ‘Tumours employ a whole range of physical barriers, chemical barriers and biological obstacles to an effective T cell immune response,’ he adds.
A CAR-T cell therapy developed by Maher’s group has been in Phase 1 trials against head and neck cancer since 2015. These T cells have genetically modified CARs that hunt a family of proteins called ErbB, prevalent on tumour cells. They also have engineered artificial cytokine receptors on their surface that improve the process of T cell multiplication before they are infused into the body. As ErbB is also expressed, at a lower level, in many healthy cells, this CAR-T therapy is injected directly into the tumours. ‘We have found that, by putting the CAR-T cells into the tumour, we minimise any risk of significant toxicity,’ says Maher. ‘We are also seeing encouraging data from an efficacy point of view.’ The team has dosed 18 patients with these CAR-T cells so far, and 10 had achieved disease stabilisation when followed up six weeks after administration.
Maher’s spin-out company, Leucid Bio, is developing his CAR-T cell design to further enhance safety and potency against various solid tumours. The closest one to clinical testing is a design that goes after stress molecules called NKG2D ligands. ‘When a cell becomes unhealthy or stressed for whatever reason, it tends to flag itself as being unhappy by expressing these ligands. The immune system then knows to look at that cell very carefully and decide whether or not it should be destroyed,’ says Maher. ‘We’re exploiting that innate immune surveillance mechanism with a CAR-based approach.’ A trial against ovarian cancer with this experimental therapy is planned for 2023, he adds.
CAR-T therapies against solid tumours are also being developed by Astero Klampatsa’s team at the Institute of Cancer Research in London. These scientists are focusing their efforts on mesothelioma and lung cancer. The CAR-T cells they have designed have an added feature that protects them from tumour cells’ defences – they are engineered to express proteins that resist immunosuppression. ‘We can seal the CAR-T cells from these immunosuppressive functions of the tumour microenvironment,’ says Klampatsa. These are aptly known as armoured CAR-T cells. Klampatsa’s armoured CAR-T cells are currently being tested on cancer cells and animals in the lab.
Going viral
Although all currently approved CAR-T cell therapies are for blood cancers, the concept was first developed for a different disease. The first ever CAR-T cell therapy administered to a human targeted HIV-infected cells, explains Steven Deeks, an HIV expert at the University of California, San Francisco, in the US. Deeks was involved in one of the early trials in the mid-1990s. Soon after, CAR-T work against HIV began to wind down due to the introduction of highly effective antiretroviral therapies. ‘Everyone who was doing CAR-T cell work at that time in HIV transitioned to cancer,’ he explains.
Recently, however, there has been a resurgence of interest in using CAR-T cells to treat HIV. ‘While drug therapy is effective in controlling HIV replication, many people struggle to adhere to the drug regimen, particularly those in low- and middle-income countries. The fact that there are 2 million new cases each year speaks to that effect,’ says Dropulić. ‘It’s widely recognised that a curative therapy or a vaccine is the best long-term solution for this disease.’
Deeks is leading a soon-to-start Phase 1/2 trial of CAR-T cells for HIV that Dropulić and his colleagues have developed at Caring Cross. ‘That study has been approved, but we have yet to enrol anyone,’ he says.
HIV particles hijack T-helper cells, using their replication mechanisms to reproduce. Virus-infected T helper cells display HIV envelope proteins on their surface. In the first generation of CAR-T cell therapies against HIV, these envelope proteins were the sole target. Early trials, however, found that these modified T-cells were themselves highly susceptible to HIV infection. Caring Cross looks to address this problem with its new design. The engineered T cells have two CARs that target different spots on the HIV-1 envelope glycoprotein. The first CAR recognises HIV particles on infected T-helper cells, as before. The second inhibits HIV’s ability to infect the CAR-T cell itself. Tests in humanised mice showed highly promising results, says Dropulić.
Preventing personal attacks
CAR-T cell therapies may also prove helpful in the treatment of autoimmune diseases. Cabaletta Bio – a spin-out company from the University of Pennsylvania in the US – is developing CAR-T cell-like therapies for autoimmune diseases involving B cells. The main function of B cells is to produce antibodies against bacteria and viruses. In people with autoimmune diseases, however, these cells mistake healthy tissues as foreign and produce autoantibodies that attack healthy tissues and cells.
Cabaletta Bio has a CAR-T cell-like therapy in Phase 1 clinical trials against the rare skin autoimmune disease mucosal pemphigus vulgaris. This disorder causes painful blisters on mucous membranes such as those in the digestive, genital and urinary tracts. Although these aren’t typically life-threatening, a one-time curative treatment would significantly improve these patients’ quality of life, explains Michael Milone, who co-founded Cabaletta Bio with his Pennsylvania colleague Aimee Payne.
Groups are developing CAR-T cell therapies for other autoimmune diseases such as lupus and multiple sclerosis
T cells engineered to treat mucosal pemphigus vulgaris are a little different from standard CAR-T cells. They host autoantibody receptors on their surfaces rather than antigen receptors, says Milone. ‘The T cell now engages the surface antibody on those autoantibody-producing B cells, and hopefully kills them in a very specific way. So we don’t kill all B cells, we just kill the cells are making the autoantibody,’ he adds.
Cabaletta Bio is also in the pre-clinical stages of testing its CAR-T cell design against other rare autoimmune diseases involving B cells, including those related to the nervous system and kidneys. There are also academic groups developing CAR-T cell therapies for other more common autoimmune diseases such as lupus and multiple sclerosis.
Jumping hurdles
CAR-T cell therapies are already saving – and extending – lives, and in the future, increasing numbers of approved therapies for a variety of indications will certainly offer hope for a growing number of patients. But there are still hurdles to overcome if they are to reach their full potential.
The most significant one is cost. ‘These therapies are very expensive and can cost in excess of $350,000 [£260,000] per dose,’ Dropulić explains. ‘It’s going to be very difficult to sustain such high pricing in the developed world, let alone in low- and middle-income countries.’ Caring Cross is developing a place-of-care manufacturing process as a means to cut costs. By eliminating the need to cryopreserve and transport cells to centralised manufacturing facilities, Dropulić believes CAR-T cells can be produced in hospitals for a tenth of the current cost.
There’s a great deal of interest in off-the-shelf approaches
The GMP process uses the benchtop CliniMACS Prodigy instrument, sold by Miltenyi Biotec for various cell processing applications, to create CAR-T cells from patients’ T cells. Caring Cross recently reported positive Phase 1 clinical trial results using experimental CD19-targeting CAR-T cells made this way for treating both ALL and B-cell lymphoma. The manufacturing process took just eight days.
The localisation of manufacturing is not the only avenue being explored to reduce the high cost of CAR-T cell therapies. Another approach would be to create universal CAR-T cells that can be infused into anyone. ‘There’s a great deal of interest in going for off-the-shelf approaches whereby you take cells from a healthy donor and manufacture a batch of drugs which can then be used to treat 50 patients, for example,’ describes Maher. ‘We’re making a bank of cells that are pre-manufactured and then cryopreserved,’ says Waseem Qasim, a gene therapy researcher at University College London. ‘The cells can be woken up by putting them in a water bath at the bedside and injected into peripheral venous system.’
The biggest challenge for universal CAR-T therapies is genetically engineering the CAR-T cells to overcome rejection in the body. This is being attempted using a variety of targeted gene-editing tools including transcription activator-like effector nucleases (Talens), Crispr–Cas9 and base editing. A lentiviral vector delivers the genetic tools necessary for the expression of CD19-targeting receptors and also to complete the gene editing needed to suppress rejection.
A number of Qasim’s patients have received experimental universal CAR-T cell therapies at his paediatric immunology clinic in Great Ormond Street Hospital. The first trial started in 2016, and was a Phase 1 trial of a Talens-edited universal CAR-T cell therapy as a drug-of-last-resort against ALL. Servier and Allogene are co-developing this treatment. Seven children and 14 adults were treated across several sites. Over 6 months, 55% of patients had survived and 27% showed no signs of cancer progression. Phase 1 trials of this therapy are ongoing, although not at Great Ormond Street.
In his clinic, Qasim is now conducting a Phase 1 trial of a Crispr-edited universal CAR-T cell therapy – again against ALL. Patients began enrolling in August 2020. His team are also planning its first Phase 1 trial of a universal CAR-T cell therapy using base editing, this time against T cell leukaemia. ‘I’m hoping [this trial] gets underway later this year,’ says Qasim.
The third method of smoothing the process and reducing the costs associated with this kind of therapy is to convert the T cells into CAR-T cells while they are still inside the body. This avoids the complexity involved with making these cell therapies ex vivo. This is a very active research area, but so far no in vivo CAR-T cell therapies have yet been tested in humans.
There is so much activity in this field it is hard to keep up
Making sure the lentiviral vector or other delivery vehicle transports the genetic material exclusively to T cells is the most challenging aspect for in vivo CAR-T cell designers. June and his Pennsylvania colleagues, Jon Epstein and Drew Weissman, are using lipid nanoparticles decorated with antibodies that seek out the transmembrane glycoprotein CD5 found on T cells for this purpose. Weissman developed this mRNA delivery method, which is also used in the Covid-19 mRNA vaccines.
In contrast to all the ex-vivo CAR-T cells, those produced by this group in vivo are not permanent. The mRNA that carries the genetic instructions for the CAR, which targets the fibroblast activation proteins expressed some fibroblast surfaces, lasts only a few days inside the T cells. After that, the T cells return to normal. This transient nature of this design particularly suits the treatment of fibrosis, better known as scar tissue.
Transient CAR-T cells are essential for treating fibrosis, as the body needs to retain the ability to create useful scar tissue in the future, for instance, for healing wounds and cuts. Epstein is a cardiologist, so their initial focus is on fibrosis in the heart. ‘Almost every form of heart failure comes along with a lot of scar tissue in the heart,’ he says. He foresees transient in vivo CAR-T therapies also being used to treat fibrosis in many organs and tissues for which there are few effective therapies. In a mouse model, the scientists have demonstrated that their approach drastically reduces the number of cardiac fibroblasts and restores heart function. Further preclinical studies are ongoing.
A decade after Emily became the first paediatric patient to receive a CAR-T cell therapy, this type of gene therapy is providing a cure to a growing number of cancer patients for whom all other treatments have failed. The technology is still also evolving to become more potent, cheaper and to cure a growing number of diseases. ‘There is so much activity in this field it is actually, at this point in time, hard to keep up!’ Milone concludes.
Nina Notman is a science writer based in Salisbury, UK










No comments yet