From brain cells to batteries, is there anything focused ion beam–scanning electron microscopy can’t study? Emma Davies takes a look
‘I like to answer questions. If someone tells me something is not possible, I usually get interested.’ Andreas Schertel is up for any challenge involving microscopy. Working in the customer centre at German microscopy company Zeiss, he has helped find ways to analyse all sorts of things, from shower gel to mouse brains, zebrafish tails and sea urchin embryos.
Schertel’s speciality is focused ion beam–scanning electron microscopy (FIB–SEM), originally targeted at the semiconductor industry but now used in an astonishing array of applications. FIB and SEM have been used separately for decades but researchers are increasingly drawn to the combined technique, which brings both high-resolution imaging and micromachining.
The ion beam is usually produced from liquid gallium and can be used for imaging or to carve away ultra-thin slices, while the SEM analyses each fresh layer. Stacking the SEM images then creates a three dimensional picture of what lies below the surface. When the SEM’s electron beam scans across a sample, different information can be gathered from emitted secondary electrons, backscattered electrons and x-rays. For example, secondary electrons give information about the surface as well as electrical properties.
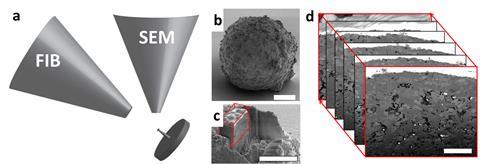
Ejecting secondary electrons from inner electron shells leaves behind positively charged ‘holes’ which are then filled by electrons from outer shells. The difference in energy between the shells is released as an x-ray, measured using energy dispersive x-ray spectroscopy (EDS or EDX).
Electrons are also backscattered from deeper within the sample, revealing elemental make-up. Generally, elements with a high atomic number backscatter electrons more strongly than light elements, thus appearing brighter in images.
Brain training
In 2006, Sriram Subramaniam from the National Cancer Institute in Bethesda, US, was one of the first to show that FIB–SEM could be used to produce 3D volumes of biological samples set in resin blocks. A few years later, Schertel used FIB–SEM to count the number of synapses in sections of mouse brains, together with a team from Spain’s Technical University of Madrid. The Spanish team still uses the technique, building detailed 3D models from images produced from many FIB-cut layers.
The work helps studies of diseases such as Alzheimer’s, explains Madrid researcher Javier DeFelipe. For example, it allows them to compare the distribution of synapses in diseased and healthy brains. ‘We are trying to build the puzzle. We hope to be able to fill the gaps and to better understand the working of the brain,’ says co-worker Angel Merchán-Perez. ‘The importance of this is that we can quantify all the data. You can give very accurate estimates of the density of synapses, their size and their distribution,’ he adds. Knowing how many neurons and excitatory or inhibitory synapses are in a brain section also helps to build accurate predictive models.
In a similar study, a US and UK team has used FIB–SEM to understand how neurons attempt to repair themselves after spinal cord injury. Surviving neurons try to re-establish pathways, and glial cells are thought to play a critical role in reconnecting neural circuits, clustering around motor neurons. The cells are believed to regulate signalling mechanisms that control neural repair ability yet it has not yet been possible to see this happening.
We hope to be able to fill the gaps and to better understand the working of the brain
Angel Merchán-Perez, Technical University of Madrid
A team led by David McComb from Ohio State University in the US has worked with Philip Withers from the University of Manchester, UK, to fill the knowledge gap by combining x-ray computed tomography (CT), FIB–SEM tomography and scanning transmission electron microscopy (STEM).
They fixed a mouse spinal cord in resin and used x-ray CT to locate regions of interest, before using FIB to trim the sample and FIB–SEM to examine it. The 3D results confirm earlier studies suggesting that direct interactions between glial cells and neurons are likely to be key to neural repair.
Developing the correlative approach is useful in itself. ‘The workflow can be applied to a terrifically wide range of problems, from looking at a thermal barrier coating on metallic components in gas-turbine engines to the interaction of a prosthetic implant with mineralised tissue such as bone,’ says McComb, director of Ohio State University’s Center for Electron Microscopy and Analysis.
Soft samples
Schertel set himself the challenge of imaging biological samples in their native state in 3D, opting to freeze them rapidly under high pressure. The cryogenic technique avoids dehydration, staining and embedding steps so that imaging can begin immediately after freezing. Samples are frozen so quickly there is no time for ice crystals to grow; they are vitrified.
In 2011, Schertel worked with a team led by Wiebke Möbius from the Max Planck Institute of Experimental Medicine in Gottingen, Germany, to analyse mouse optic nerves using cryo-FIB–SEM. The 3D imaging showed up highly detailed structures inside the cells’ mitochondria, including nuclei and nuclear pores. The team discovered that contrast was best achieved by detecting low energy secondary electrons influenced by the surface potential.
Working with a team of researchers led by Lia Addadi and Steve Weiner from the Weizmann Institute of Science in Israel, Schertel has also used cryo-FIB–SEM to study zebrafish larvae and sea urchin embryonic skeletons, both widely used in developmental biology and toxicology. Bones form though highly unstable precursor mineral phases which can be altered during conventional sample preparation but are preserved by fast freezing.
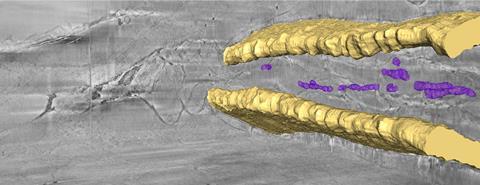
In a bid to understand how the organisms make and transport biominerals, the researchers have frozen and analysed urchin embryos and whole tails of zebrafish larvae. Backscattered electrons clearly show the bones and localised areas of biominerals, while secondary electron detection provides information about cellular structure.
‘Cryo-FIB–SEM is the only technique that can provide a 3D reconstruction of the intact hydrated tissue, with high resolution, over tens of micrometres,’ says Addadi. ‘Seeing a large intact volume allows us to understand the pathways of mineral deposition.’ In particular, they found that calcium carbonate and calcium phosphate have different contrasts when imaged using secondary electrons, although they have yet to work out why this might be.
Cool catalysts
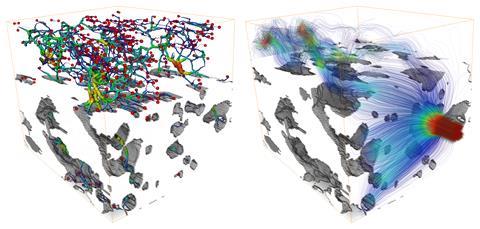
At Utrecht University in the Netherlands, Bert Weckhuysen is trying to figure out how to improve zeolite-based commercial catalysts or create new ones. A catalyst’s performance depends not only on the properties of the active sites but also on how well reactant molecules travel through its pore space towards them. Pore accessibility is not an easy property to examine in individual catalyst particles, he explains.
Using 3D FIB–SEM, his team has analysed the pore structures of various industrial catalyst particles. The results show striking differences in pore structure as well as a lack of uniformity. For example, one of the fluid catalytic cracking particles clearly has a denser surface layer, reducing access to inner pores, which would affect performance.
‘The work showed spatially distinct parts that have different porosity and activity,’ says Weckhuysen. ‘It would be cool if we could now intervene to see if we could make, for example, a structured catalyst particle that is less porous in one place and more porous in another.’ For example, large crude oil molecules might currently only be able to enter the first 2µm of a catalyst particle. ‘Can we in the near future spatially tailor porosity and therefore accessibility and performance of catalyst particles?’ he asks.
Recharge your batteries
But tissue and other biomaterials are not the only place FIB–SEM can be of use (see Cool catalysts box). Revealing in-depth 3D structure also promises to help improve lithium-ion batteries. Although dominating the rechargeable market for portable electronics, the batteries’ limited lifespan and flammability issues still leave much to be desired.
Timo Bernthaler at Aalen University in Germany has used FIB–SEM to analyse a commercial battery’s cathode, cutting out a small section for analysis. They are generally made from a lithium transition metal oxide such as lithium manganese oxide. During charging and discharging, lithium ions travel between electrodes.
X-ray microscopy revealed bright spots below the surface, while secondary electron and backscattered images from FIB–SEM showed a dark phase and a bright additive. EDS results showed the particles with the brightest contrast to be rich in lanthanum, not manganese as expected . The lanthanum is a bit of a mystery, although Bernthaler is certain it was added deliberately.
Alexander Korsunsky, who runs a multi-beam laboratory for engineering microscopy at Oxford University in the UK has used synchrotron x-rays and 3D FIB–SEM to study lithium-ion batteries, in a bid to help prevent dwindling performance after numerous cycles of charging and discharging. He follows the ‘damage cascade’ down the scales, from millimetres to Ångstroms, to understand how batteries lose their capacity.
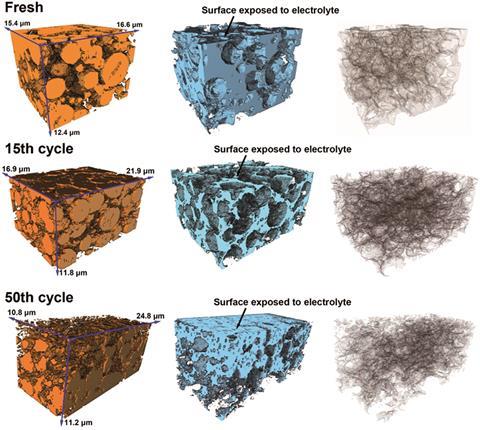
His team has analysed cathodes from both fully charged and discharged LIBs, working with Li Lu from the National University of Singapore. Their work suggests that lithiation and delithiation cause structural changes in the cathode, with expansion in one direction and contraction in the other. ‘The cycling has a mechanical element,’ says Korsunsky.
EDS is the most common SEM tool yet it falls short for analysing lithium-based systems, he says. The problem is that the detector generally sits in a chamber with a beryllium window which has very low absorption for x-rays but nevertheless cuts off some of the signal from light elements such as lithium. ‘Unless you go “windowless”, you can’t get a signal for lithium. This is a problem because everybody would like to know how lithium diffuses during charging and discharging of batteries,’ says Korsunsky.
‘We realised that even though we cannot pick up the characteristic x-ray emission we can use FIB to cut some ions from the surface,’ he says. Using the FIB’s rastering mode, the researchers sputter material away, generating secondary ions from an increasing depth in the specimen. They then detect the lithium using time-of-flight secondary ion mass spectrometry.
They have found lithium ‘hot spots’ at grain boundaries within particles. Internal defects and boundaries are likely to allow lithium ions to become trapped, they suggest. ‘If you have hot spots of lithium, you get local strain and stress,’ says Korsunsky. Using FIB tomography, the researchers found that the electrodes eventually begin to fragment.
The consequences of the hot spots and local changes in concentration are that particles that are meant to store lithium progressively fall apart, he explains. Using smaller primary particles and reducing the occurrence of internal grain boundaries could reduce the effects of lithium trapping, suggest the researchers. Despite numerous experimental challenges working with liquid electrolytes, the team has already begun to study charging and discharging in situ, says Korsunsky.
His colleagues in Singapore are currently working on solid-state batteries, which would overcome the problem of liquid electrolytes’ flammability. The researchers are starting to commercialise the technology, which ‘could be revolutionising’, he says. Korsunsky’s team is involved in 3D imaging the new devices using FIB–SEM and x-ray tomography.
Corrosion and cracks
Withers has also studied lithium-ion batteries and witnessed the contents expanding and contracting during charging and discharging. ‘The batteries breathe,’ he says. His team uses correlative microscopy – an array of microscopy techniques operating at different resolutions – to solve all matter of problems, from peering inside developing chrysalises to following cracks in alloys. ‘So you might use optical microscopy to look at low length scales, x-ray microscopy for medium length scales and electron microscopy for fine length scales,’ says Withers.
We can see how the cracks nucleate, how they connect up and eventually lead to failure
Philip Withers, University of Manchester
Much of his work is in 3D. ‘One of the challenges of 3D imaging is that often the area of interest isn’t on the surface,’ says Withers. ’With traditional correlative microscopy you see something on the surface and all you have to do is find the same area using the next technique. When that area of interest lies maybe tens of millimetres beneath the surface, it’s a bit like digging for treasure.’
However, the beauty of 3D imaging is that Withers is able to study cracks as they grow. ‘By studying the same sample, we can actually see how the cracks nucleate, how they connect up and eventually lead to failure.’ His team typically uses x-ray imaging to find a crack. ‘We may want to look at the damage in front of the crack because it gets joined up, a bit like joining up the dots.’ For this, the researchers can cut out the region and do FIB serial sectioning to image in high resolution.
He has used the technique to study corrosion in stainless steel, cutting layers away under a corrosion pit using FIB–SEM, all the while imaging with electron backscatter diffraction. Together with STEM results, the images reveal a clear ‘corrosion front’ moving along grain boundaries. EDS also shows that elements in the alloy segregate ahead of the crack.
In the navy
Understanding how materials in ships and aircraft behave in salt water and other corrosive environments is particularly important for the navy, helping to build stronger structures and save on repairs. At Arizona State University, US, the US Office of Naval Research (ONR) and Zeiss part-fund a new Center for 4D Materials Science.
The centre studies materials in the three spatial dimensions and over time (the fourth dimension) using techniques that span the length scale from nanometre to micrometre and beyond . ‘The idea is to use this correlative approach to come up with answers to problems that we have not been able to solve,’ says Nik Chawla, who heads the centre.
‘The ONR has an interest in how the materials on their ships, aircraft and other structures behave in salt water and corrosive environments,’ he says. Since aluminium alloys are ubiquitous, understanding how corrosion pits nucleate and grow is important, he adds.
It’s a great technique but it’s not the solution for everything
David McComb, Ohio State University
For example, his team has been running real-time experiments on aluminium alloys using x-ray CT and FIB–SEM. They have found magnesium-rich precipitates and discovered that these areas dissolve preferentially in contact with corrosive fluid, whereas iron-based precipitates and particles are left untouched.
Challenges
Despite its obvious appeal and innumerable applications, FIB–SEM is not a perfect tool. ‘It’s a great technique but it’s not the solution for everything,’ says McComb. ‘For example, for the work we did on the spinal cord, we reconstructed a volume that was 100×100×100µm. To acquire the data alone took us about 60 hours,’ he says. A normal FIB–SEM is not designed to go through large volumes of material, he adds.
In some cases, plasma FIB (PFIB), which uses argon or xenon instead of gallium, can be used for larger volumes. In Manchester, Withers uses PFIB–SEM to mill samples at high rates, 60 times higher than gallium-based systems. He suspects that there are ‘many cases’ where features hundreds of microns beneath the surface can be identified using x-ray CT and analysed using PFIB–SEM.
Just don’t mention the data. Storing, analysing and visualising high resolution 3D datasets pushes computer systems to the limit and requires unlimited amounts of the most valuable resource of all: time.
Emma Davies is a science writer based in Bishops Stortford, UK











No comments yet