Spurred by advances in energy-harvesting materials, a new generation of advanced implantable biomedical devices is emerging that does away with the bulky battery. James Mitchell Crow reports
Wireless power transfer may seem like a recent innovation to keep energy-hungry smartphones topped up, but for biomedical electronics, wireless power transfer has a deep history.
When Swedish doctors performed the first fully implantable cardiac pacemaker surgery in 1958 , the device they had invented was powered by a rechargeable nickel–cadmium battery, connected to a wire coil that enabled magnetic induction wireless recharging across the skin. The battery, coil and controlling electronics were housed in a shoe-polish-sized can implanted in the abdomen, with leads running to the heart to deliver the pulsed electrical stimulus.
Millions of people with a slow or irregular heartbeat have since benefited from a pacemaker implant – which soon evolved to use longer-lasting batteries that were replaced surgically every few years, rather than requiring a weekly recharge. More than six decades later, however, the basic pacemaker design – a remotely located can containing the bulky battery pack and electronics, connected to the heart by long leads – remains unchanged.

Of the few implantable devices that have followed the pacemaker into clinical use, even these typically follow that original design. For example, deep brain stimulation devices for conditions such as Parkinson’s and treatment-resistant depression use a pacemaker-like chest-mounted electronics and battery pack, with electrical leads running all the way up the neck to the brain.
The long wires and bulky battery packs are not just the main failure point for implanted devices, notes Jacob Robinson, who designs implantable electronic devices at Rice University in Houston, US. They have become the key constraint on next-generation biomedical implant device design, he says. ‘What prevents you from developing implant technologies that could be made extremely small, and scale to a network of nerve stimulation and recording devices, is the battery.’
As bioelectronics has advanced, experimental implants have been demonstrated that enable people with severe spinal cord injuries to walk again, or people who have lost a limb to control and experience a sense of touch from a robotic prosthetic.
To turn these experimental prototypes into practical devices that could be implanted at target sites around the body, developing miniaturised all-in-one implants could be key. The limitation on miniaturisation is not the electronics, but the power available after shrinking the battery. ‘A small battery would not support complex stimulation and recording function for a long enough time to be practical,’ Robinson says. ‘We need to find a way to provide enough energy – and that means a battery-free device.’
With innovative use of advanced materials, Robinson’s lab is one of the pioneers seeking to design a new generation of implanted biomedical devices that do away with the bulky battery and failure-prone connecting leads. The goal is to create implants that can fully satisfy their own power needs by harvesting energy locally available within the body, or via high power wireless energy transfer.
Good vibrations
Batteries don’t just limit human medical implants, but also fundamental biomedical research, says John Rogers, a materials scientist at Northwestern University in Illinois, US. As well as designing implantable medical devices, Rogers develops implants designed as advanced tools for neuroscience research, studying brain function in rodents.
‘For small animal studies, the size, weight and bulk of the battery can prevent the implantation of electronic systems,’ Rogers says. Cumbersome external battery packs don’t solve the problem as they compromise the animals’ natural behaviours, and studying social interaction is impossible because they chew one another’s hardware. ‘In those instances, it’s essential to get rid of the battery,’ Rogers says.
There are many possibilities for battery-free power. ‘People have tested pretty much everything you can think of,’ says Rogers. Implantable nanogenerator technologies trialled have ranging from tiny electricity-generating biofuel cells that run on the glucose present in the body, to thermoelectric harvesting of power from the temperature gradient between the body and the outside world.
Triboelectric generators can be made from materials that are very flexible, biocompatible, even biodegradable
‘You can also think about harvesting power from natural mechanical motions of the body, such as the beating of the heart, inflation and deflation of the lungs, or the kinematic motions of the limbs,’ Rogers says.
From a materials perspective, there are two main ways to harvest energy from muscle-driven motion, says nanomaterials researcher Xudong Wang, who develops implantable electronic devices at University of Wisconsin–Madison, US. One exploits triboelectricity, a surface effect that generates an electric field when two materials in contact move relative to each other to induce charge separation. The effect is maximised when an electron donor and an electron acceptor material are paired – but beyond that, there’s an almost limitless array of materials that can be used.
‘Triboelectric generators can be made from materials that are very flexible, biocompatible, even biodegradable,’ Wang says. The downside of a triboelectric generator is the requirement to combine materials in a device that allows their individual movement. ‘That brings design challenges, particularly in very confined spaces inside the human body, where the displacement is relatively small.’
Piezo power
The alternative is to harvest energy from body motion with piezoelectric materials, which generate an electric field when placed under mechanical strain. ‘Piezoelectric nanogenerators use a single piezoelectric material, so we don’t need to consider any relative movement inside the device,’ says Wang. ‘In locations in the body where we cannot accommodate much local movement, piezoelectric materials are a better choice.’
Wang’s lab explores biocompatible piezoelectrics made from biomaterials, such as crystalised thin films of amino acids. ‘These materials are non-toxic and sustainable, don’t need toxic chemistry to produce, biodegrade naturally in the environment – and can provide considerable energy conversion efficiency,’ Wang says.
In 2021 , the team reported a way to grow piezoelectric thin films made from glycine. In pure form, the amino acid forms a brittle film in which the glycine molecules are randomly aligned. ‘For the piezoelectric device to be useful, we need ordered alignment so that all the polarisation is on the same side, just like we have to install batteries in the same way to get high output.’
Wang solved the issue using a method called surface interface directed crystallisation. ‘We added a water-soluble polyvinyl alcohol polymer together with the amino acid,’ he says. ‘The polymer serves as a template, providing a lot of hydroxyl groups, which link with glycine specifically so that all the glycine molecules arrange in the same way.’
The polymer also forms a protective coating that enables the glycine layer to flex without breaking. ‘We could use the flexible film in an implantable energy-harvesting device.’ In rats, the team showed they could place the device in the thigh to generate power from walking, or in the chest to generate power from breathing.
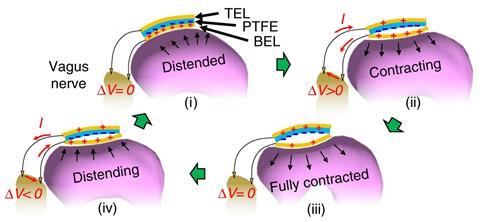
One application Wang is investigating is as a potential treatment for obesity, which he has successfully demonstrated in animals. ‘We developed a device that we put on the surface of the stomach, and wire to the vagus nerve,’ he says. The vagus nerve connects the digestive system to the brain. ‘When the animal eats, the peristaltic movement of its stomach generates electric pulses from the device, which pass into the vagus nerve and provides a signal to say ‘Stomach is full.’’ Rats with the implant ate one-third less food, and weighed almost 40% less, than untreated animals.
Picking up the pace
For today’s cardiac pacemakers, a significant downside is the need for regular surgery to replace the device each time the battery runs low. Self-powered pacemakers that use energy harvesting to continually capture power from the heartbeat could remove that need.
Flexible piezoelectric materials attached to the heart’s outer surface have been one avenue of exploration. A team in China recently tested an alternate design, an all-in-one capsule-shaped device attached to the inner wall of a heart chamber. As the heart beats, polyformaldehyde pellets inside the capsule roll back and forth between polytetrafluoroethylene-coated gold electrodes at either end of the capsule. The motion produces an alternating current due to triboelectricity and electrostatic induction. In pigs, the device captured enough energy from every four heartbeats to power a pacing pulse. The team aims to improve the device to harvests enough energy from each heartbeat to generate a stimulating pulse.
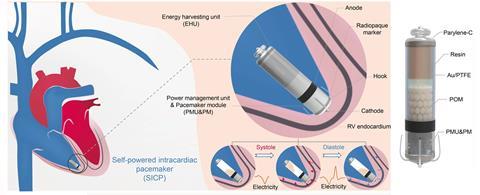
Energy harvesting materials implanted deep within the body can also power sensing applications, says Hoe Joon Kim, who leads the nanomaterials and devices lab at Daegu Gyeongbuk Institute of Science and Technology in South Korea.
One project underway in Kim’s lab is to develop a flexible piezoelectric cuff, which could be placed around a large blood vessel deep within the body. The device would be powered by, but also directly sense, the pulses of blood passing through the blood vessel. ‘We could directly monitor blood pressure near the heart, which is not really possible with existing blood monitoring systems,’ Kim says.
The team is also exploring the use of piezoelectrics or triboelectrics to support healing. Bone is piezoelectric, and the effect naturally plays a role in bone fracture healing. ‘There’s always a very minimal generation of electric charge, which really doesn’t affect our body function, but let’s say you have a crack on your bone,’ Kim says. As you move, there’s more of a flex – and so a more focused generation of electric charge – at the fracture site. This charge stimulates osteoblast activity, promoting healing.
Powerups
Energy harvesting can generate enough power for simple devices. But when Robinson considered the power demands of the more sophisticated sensing and stimulating electronics he hopes to implant, capable of wireless data transfer, he found a significant shortfall.
Whichever mode of energy harvesting you look at, biological systems have evolved for energy efficiency, Rogers says. ‘There’s not a lot of free energy floating around that you can tap into,’ he says. And trying to take too much can have extreme consequences.
Rogers’ lab previously trialled ultra-thin piezoelectric materials on flexible foils, mounted onto the heart to generate electricity from heartbeats. ‘There are estimates that put the amount of power that’s being consumed by the heart in the range of watts – and so you would say ‘Wow, that’s a lot of power, I only need a milliwatt to operate my Bluetooth radio and implanted electronics,’’ Rogers says.
There’s just not a lot of free glucose to tap into
But tapping the heart isn’t just taking free energy. The piezoelectric device harvests energy by mechanically loading the heart, Rogers says. ‘If you load the heart too much – and too much is not very much, it turns out – you will induce a spontaneous arrhythmia from which the heart can’t recover.’
Even in large animals with human-scale hearts, the team could only harvest up to a few tens of microwatts. ‘You could generate just enough to operate the most power efficient pacemaker, but you’re far short of what would be needed for wireless communication, for example,’ Rogers says.
Physical motion such as walking offers more power, but too intermittently for most applications. Thermoelectric power harvesting from the body is even more challenging to capture enough energy, Rogers adds. ‘With biofuel cells it’s the same story, there’s just not a lot of free glucose to tap into.’
To develop practical systems with real world functionality, Rogers’ research is now focused on wireless power transfer. Rogers has primarily explored wireless transfer via magnetic induction, using transmitter and receiver coils to deliver energy into the body. ‘You can deliver tens of milliwatts no problem, operating over ranges of up to a metre or so,’ Rogers says.
Melting away
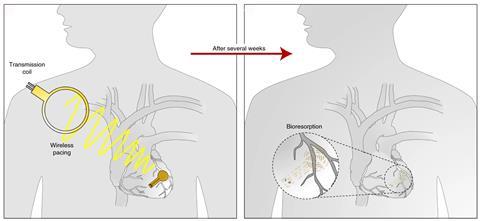
For human health care applications, Rogers has developed wireless power transfer implants made from materials that are fully bioresorbable once the device has completed its task. ‘We got contacted by cardiac surgeons who identified a possible application of that technology for patient care following open heart surgery,’ Rogers says. Currently, clinical standard practice post-surgery is to insert a temporary pacing lead that passes out through the skin to connect to an external power supply. As the patient recovers during the first few weeks after surgery, the heart can be paced if the heart rate drops below a threshold level.

Once the patient has recovered, the pacing lead can be pulled out. But in up to 5% of patients, pulling the lead free tears the healthy cardiac tissue, causing a potentially fatal internal bleed, says Rogers. ‘The surgeons’ idea was to make a wirelessly powered, wirelessly controlled, bioresorbable temporary pacemaker, which would just melt away after a couple of weeks to eliminate the need for removal.’
For bioresorbable conductive wiring, the team had a range of metals to play with. ‘Iron, zinc, tungsten, molybdenum and magnesium are all essential elements for healthy metabolism, and all great electrical conductors,’ Rogers says. In contact with biofluids in the body, they undergo hydrolysis to form soluble hydroxides that are eventually expelled from the body. For the semiconductor components, ultra-thin silicon is a biocompatible and bioresorbable.
The device is encapsulated in a biocompatible synthetic or biologically occurring polymer such as collagen or polycaprolactone. To control device lifetime, the team tunes the surface chemistry of the encapsulation layer. ‘Generally, we like hydrophobic polymers that undergo surface-erosion-based biodegradation chemistry,’ Rogers says. ‘We design a device to last twice as long as it really needs to last for clinical application, and then it just dissolves away over time.’
The team designed the implant to pair with an external chest-mounted skin patch containing the power transmitter and Bluetooth for wireless data transfer. ‘It can operate in a closed loop fashion, sensing the heart rate and, if it drops, activating the pacemaker to boost the heart rate back up to a healthy set point,’ Rogers says.
Combined harvesters
As with energy harvesting, there are multiple ways of delivering power wirelessly into the body – sometimes using the same materials. ‘Ultrasound is another option,’ Rogers says. ‘You can propagate ultrasonic waves into the body, get good penetration, and use piezoelectric harvesting components to capture power from those ultrasound waves.’
Robinson was initially drawn to ultrasound-based wireless power transfer, rather than magnetic induction, because of the miniaturisation potential it allows. ‘The size of my receiver can be much smaller than a comparable electromagnetic receiver,’ Robinson says. But ultrasound power transfer is sensitive to misalignment between the ultrasound beam and the receiver. And as with ultrasound for medical imaging, the transmitter needs to be in direct contact with the skin, because ultrasound passes poorly travel through the air. ‘We started thinking, what if there was a way that we could have the benefits of ultrasound, but without having to directly couple with a propagating ultrasound wave?’ Robinson says.

The solution was inspired by an obscure paper in the literature describing magnetoelectric materials, Robinson says. ‘Magnetoelectrics vibrate at the same frequency as ultrasound waves, but in response to a magnetic field rather than an ultrasound beam,’ he says. To create a functional device, the team created a magnetoelectric metamaterial by laminating together a magnetostrictive material with a piezoelectric material. The magnetostrictive material vibrates in response to the alternating magnetic field, and the piezoelectric converts the vibration into an electric field.
‘By varying the thicknesses ratio of the two materials, and testing new ways to laminate the materials together, we were able to improve power density by five times, and break the record for energy per millimetre squared in bioelectronic devices,’ Robinson says. Magnetoelectrics also offers a novel mode of wireless data transfer as well as power transfer.
The team has packed their magnetoelectric metamaterial into a tiny self-contained brain stimulation device small enough to be placed within the bone cavity created by drilling a standard 14mm burr hole in the skull. Potential applications include deep brain stimulation for movement disorders and psychiatric conditions.
‘We spun out a company out of my lab to build devices small enough to be implanted in the skull in about a 20-minute procedure,’ Robinson says. The company is targeting 2025 to apply to the US Food and Drug Administration (FDA) to conduct a clinical trial.
Rogers has also spun out a company, to advance his bioresorbable temporary pacemakers toward clinical trials. ‘The FDA has never examined any temporary electronic technology like this before,’ Rogers says. The key question will be to evaluate how the device breaks down once its job is complete, he adds. ‘On the benchtop they melt away in a very controlled manner, but inside living animals there is all kinds of motion, so it dissolves to a certain level and then fragments.’
Again, materials science could solve the problem. ‘You could encapsulate the whole thing in a hydrogel that dissolves on a longer time scale than the constituent materials,’ Rogers says. The hydrogel would contain all the fragments until they fully break down, before itself bioresorbing. ‘Those are the kinds of issues that you really have to look as a first critical step before first in human use.’
It’s a different world from the unregulated experimentation of the 1950s, when an experimental device could just be popped into a willing heart patient. But it also continues the innovative use of materials science to solve unmet medical needs.
‘One of the really exciting things about our magnetoelectrics discovery was that data and power transmission was now mediated by material rather than an antenna,’ Robinson says. ‘And so now there’s a material science opportunity to make better and better devices.’
James Mitchell Crow is a science writer based in Melbourne, Australia

















No comments yet