Learn to control chemistry using electric fields and the consequences could be revolutionary, says Joshua Howgego
Chemists are used to harnessing all sorts of subtle and not-so-subtle tools to choreograph the dance of molecules, from lasers to microwaves to plain old heating and stirring. But now, in a few labs around the world, an unusual new idea is crackling and sparking into life: chemists are starting to explore whether electric fields can be used to control reactions too.
At first sight, an electric field might seem like an untameable partner; could such a diffuse force really be used to influence the making and breaking of individual bonds? But a stunning experiment conducted early in 2017 showed for the first time that this is precisely what can be done. Set things up just right, and these fields can conjure chemistry in supercharged style. Now the race is on to see where this control could take us.
Bright idea
The spark for this revolution came from Sason Shaik in 1974, when he was sitting in a small, dim lecture hall at the University of Washington in Seattle, US. The lecturer was a man who had a habit of waving his arms and hands around to act out the motions of atoms and molecules. ‘This was very vivid, so I always listened attentively,’ says Shaik. On this particular day, the lecturer was describing a catalyst: a molecule that can speed up the rate of a reaction without itself getting used up. But not any old catalyst; this one sped up a reaction a million times. ‘I was pretty impressed,’ says Shaik, who is now at the Hebrew University in Jerusalem, Israel. ‘I thought: this is like the best of the best.’
What Shaik had heard about was a reaction involving tertiary butyl chloride. Dissolved in ether, only a tiny fraction of the molecules will split at the carbon-chlorine bond. But add a high concentration (5.5M) of a simple salt, lithium perchlorate, and the rate of bond-breaking goes up a million fold.
Shaik never got to the bottom of exactly how the lithium perchlorate catalysis works [but] he began to think that electric field catalysis wasn’t such an outlandish idea
It’s not such a useful reaction in itself but this simple salt catalyst did an almost unbelievably good job of speeding it up. Shaik wanted to know how. He soon realised that at such high concentrations, each catalyst molecule could only have about two solvent molecules around it. In other words, it was dominating the whole set up, and he suspected it could be setting up a liquid crystal-like matrix and perhaps introducing an electric field into the solution. It could be that electric field which drives the catalysis, he thought.
Shaik never got to the bottom of exactly how the lithium perchlorate catalysis works; his focus was on building a research career studying reactivity using valence bond theory. But the problem did stick in his mind and he began to think that electric field catalysis wasn’t such an outlandish idea.
Imagine, for example, a hydrogen molecule being approached by a cation along its bond axis. The electric charge of the cation polarises the molecule, forcing more of the electron density towards the far hydrogen atom. Continue the thought experiment and eventually the bond would break – chemistry would have happened. That’s not controversial. The question is, can you do the same thing with an external electric field?
In 2004, Shaik decided to take this question on. He began by looking at an active part of the heme unit found in many enzymes, including cytochrome P450, which is crucial in metabolism. This compound, known as ‘compound 1’, is a porphyrin with an FeO unit bound in the middle. There are three unpaired electrons dotted across the structure, which can take up two different arrangements. Shaik wanted to know if an external electric field could influence which of those arrangements was adopted and whether that affected the enzyme’s behaviour.
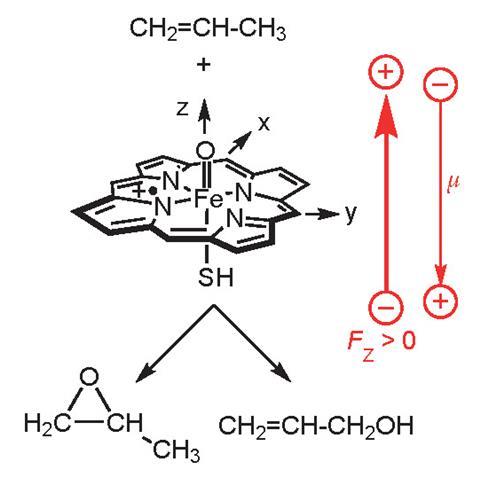
He used computer simulations to model compound 1 binding to propene with an electric field applied parallel to the Fe–O bond.1 From there, the propene could be either epoxidised or hydroxylated, depending on the arrangement of electrons. Shaik found that with the electric field applied to favour one polarity, the transition state relating to the hydroxylation was more stable by 6–10 kcal mol-1, but switch the polarity and the epoxidation transition state was favoured by 2–6 kcal mol-1. ‘All you’ve got to do is switch the field,’ he says. ‘If you change the direction, you could get either one.’ It was a neat result, which seemed to suggest that the concept was worth exploring – these fields could entirely change the fate of a reaction.
Encouraged, Shaik began to think bigger and tried modelling the effect of an electric field on Diels–Alder reactions. This was, however, something entirely different. Up until now, he had shown that an electric field could influence the stability of charged transition states, which seems reasonable. But a Diels–Alder reaction involves two molecules – a diene and a dienophile – snapping together with no intermediates involved, charged or otherwise. So when he computed that an electric field could catalyse the reaction and affect its endo/exo selectivity, he had a hard time getting the results published, only succeeding in 2009.2 After that, says Shaik, it got really tricky to publish anything. ‘So I left the field for a while.’
Jump start
Years passed but then, quite suddenly, everything changed. In March 2016, a journalist called and told Shaik that some researchers had tried his electric-field catalysed Diels–Alder reaction for real.3 Michelle Coote of the Australian National University in Canberra, who led the team responsible, had stumbled across Shaik’s work and just so happened to have the right connections to test it out.
Through a colleague, Coote had come into contact with Nadim Darwish at the University of Barcelona in Spain. Darwish conducts experiments involving scanning tunnelling microscopy (STM). When an STM tip is brought close to a conducting surface and a voltage difference is created between the two, electrons can quantum tunnel across the gap. Darwish had been using this set up to develop so-called ‘blinking’ experiments. A molecular bridge between the tip and surface changes the conductance across the gap. So moving the tip over a surface will cause small blips in conductance – blinks – to occur whenever these bridges form.
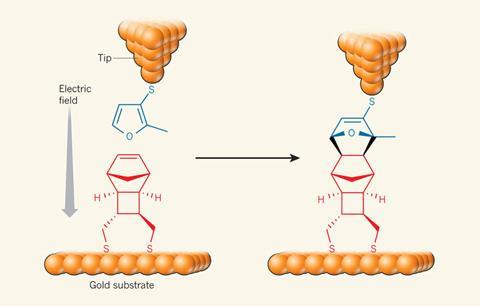
Coote, Darwish and their colleagues festooned an STM tip with diene molecules and a surface with dieneophiles. They could then move the tip along the surface and count the blips, which signalled the two molecules had joined together via a Diels–Alder reaction. But here’s the neat part: by changing the voltage gap between the tip and surface, they could ramp up the electric field experienced by the Diels–Alder reactants as they reacted. At –0.05V they saw only five blinks per hour. But at –0.75V, that increased to 25 blinks per hour. At the end of their paper, Coote and her colleagues wrote: ‘This ability to manipulate chemical reactions with electric fields offers proof-of-principle for a change in our approach to heterogeneous catalysis.’
That was quite a statement and, naturally, Shaik was delighted with the vindication. He soon wrote a perspective article for Nature Chemistry extolling electric fields as ‘future smart reagents’.4 But if you’re about to throw away your catalysts, then stay your hand for the moment.
Part of the reason Shaik sometimes had difficulty getting his ideas published was because reviewers had trouble seeing how electric fields could ever be practically useful catalysts. In Shaik’s simulations, the electric field had to be aligned with a particular axis of a molecule, yet in solution molecules tumble around all over the place meaning only a tiny fraction would be in the right orientation at any one time. Coote’s work only got around this by fixing them in place on the tip of an AFM – a beautiful demonstration but hardly a scaleable synthetic method.
Finding the flow
There may be hope of something better, however, in the shape of some kit developed in the labs of Matt Kanan at Stanford University in Califonia, US. In 2012, Kanan developed what he calls a ‘parallel plate cell’.5 It has a charmingly homemade look: two glass slides sandwich two strips of copper mesh, which in turn sandwich two metal plates coated in aluminium oxide. In the very middle is a gasket with a hole for a reacting solution. Kanan filled this with an acetonitrile solution of cis-stilbene oxide, then attached crocodile clips to the copper mesh. When the power is switched on, it creates an electric field just at the interface between the aluminium oxide and the solution.
Kanan tried playing around with the applied voltage, and leaving the reactions to run for a while before analysing the products. With no juice flowing, the ratio of aldehyde to ketone was about 1:2. When it was switched on and set to +5V, that ramped up to 10:1. Strangely, a similar effect, biased towards the same product, appeared when he tried negative voltages.
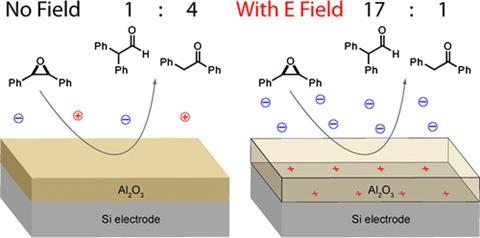
These results suggest that you can get can electric fields to catalyse reactions in situations more conventional than the inside of an STM microscope. But it’s not immediately clear how it works; it is possible that the thin layer of electric charge inside the device may encourage molecules to align in neat rows.
If that’s true, then one way to make electric field catalysis scaleable might be to convert Kanan’s cell into a flow reactor, so that the molecules flow past the electric field over and over again, increasing the probability that they eventually align perfectly with it and react. Whether Kanan has any intention of developing such an idea is not clear – he did not respond to requests for comment. And although he demonstrated similar electric field control over an intramolecular cyclisation using his parallel plate cell in 2013,6 there is no evidence of him working in the area since.
‘I’m still puzzled that it worked so well’
Stefan Matile, University of Geneva, Switzerland
But others are definitely interested. Stefan Matile at the University of Geneva, Switzerland, began looking into electric fields after reading Shaik’s review paper. ‘I think this topic is hotting up in the community,’ he says. ‘I’m really enthusiastic about doing things with electric fields.’
His first trick, published in May 2017, involves a supramolecular interaction called an anion–π bond.7 Matile takes an aromatic molecule, which under normal circumstances would bind cations because of its high electron density. But find a way to suck that density out of the ring, with electron-withdrawing substituents for instance, and he has shown the π surface binds anions instead.
It stands to reason that an electric field could influence the distribution of the electron density too, so Matile tried fixing his aromatic molecules to a tin indium oxide surface and applying an electric field. When the field is turned on, an enolate species binds neatly to the aromatic surface and a reaction occurs. Switch it off, and the catalyst stops working. ‘I’m still puzzled that it worked so well,’ says Matile. ‘But I’m planning to do more; I find it fascinating.’
One of the ideas he is working on, along with Marcel Mayor at the University of Basel, is to develop devices with several catalysts embedded in surfaces in sections. The idea would then be to apply an electric field to each section in sequence, which would attract reactants and perform a multistep synthesis simply by flicking the field on and off in different places.
In Matile’s devices, we have the beginnings of a method that could get electric field catalysis working at large scale, albeit a method that is tightly restricted because of the need to bind to the aromatic molecule. Plus, all of the reactions that electric fields have been used on so far have involved just one molecule. Bringing two together while getting the field alignment just right might be much more difficult.
But it’s hard to avoid thinking that the true potential of electric field catalysis will come out sooner rather than later. Chemistry World spoke to one chemist who was considering how magnetic fields might be used to hold molecules in the right alignment for the electric field to influence them, though the plans were at too early a stage to be fully divulged.
Coote, meanwhile, is exploring the use of a catalyst with charged functional groups to eliminate the need for an external electric field. That is an approach ‘that I believe is infinitely scalable in synthesis’, says Coote. ‘These too are switchable in the sense that you can change the pH or oxidation state and trigger a reaction.’
For Shaik, however, it’s the external electric fields that are really exciting. ‘I’ve been thinking about something I call a zipper reaction,’ he says. ‘Maybe it will eventually be possible to take a series of molecules, orient them on some support, zap them with an electric field, and have all bonds broken and made at the same time.’
Joshua Howgego is a feature editor at New Scientist
References
1 S Shaik, S de Visser and D Kumar, J. Am. Chem. Soc., 2004, 126, 11746 (DOI: 10.1021/ja047432k)
2 R Meir et al, ChemPhysChem, 2010, 11, 301 (DOI: 10.1002/cphc.200900848)
3 A Aragonès, Nature, 2016, 531, 88 (DOI: 10.1038/nature16989)
4 S Shaik, D Mandal and R Ramanan, Nat. Chem., 8, 1091 (DOI: 10.1038/nchem.2651)
5 C Gorin, E. Beh, and M Kanan, J. Am. Chem. Soc., 2012, 134, 186 (DOI: 10.1021/ja210365j)
6 C Gorin et al, J. Am. Chem. Soc., 2013, 135, 11257 (DOI: 10.1021/ja404394z)
7 M Akamatsu, N Sakai and S Matile, J. Am. Chem. Soc., 2017, 139, 6558 (DOI: 10.1021/jacs.7b02421)












No comments yet