Nina Notman finds out how liquid crystals are moving into the biotechnology and pharmaceutical toolbox
LCDs (liquid crystal displays) are the best known application of liquid crystals, but they are far from the only use for these quirky phases. Liquid crystal is a state of matter that’s a halfway-house between a solid and a liquid. The molecules in liquids have no long-range order and are able to flow, whereas a crystal has long-range order in all three dimensions and is therefore rigid. Structures containing molecules with some long-range orientational order, but not as ordered as in a solid, are liquid crystals.
There are many types of liquid crystals, but most of the ones made of organic molecules are classed as either thermotropic or lyotropic. Phase changes in thermotropic liquid crystals – from liquid, to liquid crystal, to solid and back again – depend on temperature. For lyotropic liquid crystals, phase changes depend on both temperature and the concentration of the molecules in the solvent.
LCDs use thermotropic liquid crystals, and therefore this class has been comprehensively studied. By contrast, lyotropic liquid crystals are less well understood, even though they are more prevalent in the world around us – found, for example, where soaps and detergents mix with water and even in cake batter.
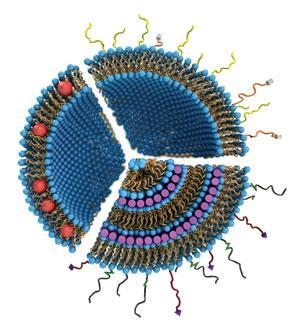
More importantly, some biological systems – including lipid membranes, RNA and DNA · are structurally similar to lyotropic liquid crystals when in water. That means the human body might find biotech devices and pharmaceuticals made of liquid crystals more palatable than those made of solid materials.
Gene therapy
One potential biotech application of liquid crystals is in gene therapy, to carry DNA or siRNA (small interfering RNA) into cells. Gene therapy is an experimental approach to treating some inherited disorders, types of cancer and viral infections. The aim is to piggyback on the way cells naturally express genes, including harmful viral genes, to replace the protein products of damaged or faulty genetic information. Cyrus Safinya at the University of California Santa Barbara, US, has been designing liquid crystalline lipid-based nanoparticles for gene therapy for the last 17 years.
Approximately 1800 gene therapy clinical trials are currently taking place worldwide. Of those, 110 have liquid crystalline carriers, explains Safinya. The majority of the trials use viral carriers that have been reengineered to make them safe, an approach with well-documented problems: a tiny minority of trial participants have had massive immune responses to the engineered viruses, some of which have been lethal, and viral carriers are much less practical to mass-produce. The synthetic carriers are going to be much easier and cheaper to make and get regulatory approval for in the long run, Safinya explains. ‘At the end, when gene therapy comes out in tablet form, it’s going to be something that’s synthetic.’
The carriers designed in Safinya’s labs are lipids that – when mixed with pieces of DNA – spontaneously form lyotropic liquid crystalline nanoparticles due to electrostatic interactions. ‘Because DNA is negative and the lipids are positive, they spontaneously self-assemble and the result of that assembly is a liquid crystal phase,’ he explains.
The idea behind gene therapy is to get the therapeutic gene into a cell nucleus so that it can be expressed, but it is surprisingly difficult to mimic viruses in this way. Getting the lipid nanoparticles into the cell is easy. ‘If you take almost any particle of a given size and dump it on a cell membrane, the cell will just naturally engulf it and take it in through a process called endocytosis,’ explains Safinya. Ensuring the lipid nanoparticles target the right cells is also achievable; his team attaches synthetic polymer chains, tipped with short peptides, to the head groups of the lipids. These peptides can recognise, and attach to, receptors on the surface of target cells.
Getting the lipid nanoparticles to move from the endosome– where they initially go upon entering the cell – into the nucleus, where the therapeutic gene can be expressed, is more difficult. ‘Escaping the endosome is one of the bottlenecks of this whole field,’ explains Safinya. The pH of the endosome when the lipid nanoparticles first enter is around 7, and over time it becomes more acidic. Safinya’s team is therefore designing polymer–peptide chains (the ones attached to the lipids for targeting) that fall apart at low pH. ‘Once the [liquid crystal] vesicle has no polymer on it, it can then start interacting with the endosome again and typically it can fuse it and escape.’ While this trick does work, Safinya concedes that it isn’t yet perfected, and nor is any other approach tried by other research groups.
DNA and siRNA aren’t the only cargoes suitable for these lipid carriers: they can also carry drug molecules. Cancer chemotherapy drugs such as paclitaxel (Taxol) could potentially be administered this way. ‘A significant advantage of delivering paclitaxel via lipid particles is the possibility of delivering it primarily to targeted diseased cells. This would improve effectiveness of drug delivery and lower toxic side effects [caused by] the drug going to healthy cells as well,’ he says. A lipid nanoparticle containing paclitaxel – referred to as EndoTag-1– is currently in clinical trials.
Anticancer drugs
With paclitaxel, the liquid crystals are carrying the active drug molecule. But materials chemist Atsushi Yoshizawa at Hirosaki University in Japan is preliminarily exploring the cancer-killing properties of liquid crystals themselves. An increasing number of pharmaceuticals are known to form liquid crystalline states, including the non-steroidal anti-inflammatory drugs fenoprofen (Nalfon; for arthritis) and cromoglicic acid (Intal; for asthma). But these are liquid crystalline by accident, not design, and it is not known if the liquid crystalline phase plays any part in their biological activity.
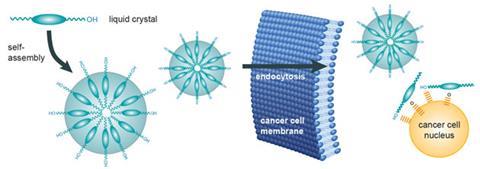
By contrast, Yoshizawa is purposefully aiming to make a liquid-crystalline anticancer drug because he believes drug molecules in a liquid crystal phase should perform better than their crystalline analogues. ‘A drug must penetrate multiple layers of solid cancer cells,’ says Yoshizawa. ‘We surmise that a supramolecular assembly consisting of liquid-crystalline molecules possessing a biologically active site is effective not only for cell permeability but also for cytotoxicity against tumour cells.’
Again he is taking advantage of the way some molecules spontaneously self-assemble into spherical nanoparticles, this time comprised of thermotropic liquid crystals. Over the last 10 years, his team has synthesised a range of molecules containing a flexible hydroxyl group attached to a core of either biphenyl, phenylpyrimidine or phenylbenzoate, and shown that they aggregate into spherical liquid crystalline nanoparticles in solution.
The team has tested these liquid crystals in vitro against a range of cancer cells including human lung cancer, colon cancer and leukemic cells, and shown that they do indeed suppress cell growth. This work is still in the very early stages, says Yoshizawa, but they do eventually hope to test the compounds in vivo.
As with Safinya’s set-up, Yoshizawa plans to ‘design an intelligent molecule that recognises differences between cancer cells and healthy cells’. These liquid crystals are taken up by endocytosis, but it is not completely clear why they kill cancerous cells. Once inside the cell ‘the molecules might interact with the nucleus via the hydroxyl group’, says Yoshizawa.
Various compounds containing multiple hydroxyl groups (known as polyhydroxy compounds) including the chemotherapy drug doxorubicin show anticancer activity, he explains. ‘Doxorubicin intercalates between the base pairs in DNA and then hinders its duplication. Hydrogen bonding between the [drug’s] hydroxyl group and the bases plays an important role in the interaction.’ Yoshizawa suspects that, once inside the cell, his team’s monohydroxy molecules change their assembly from the spherical nanoparticle, needed to enter the cell, into ‘a molecular assembly that behaves as a quasi-polyhydroxy molecule’.
Orthopaedic tools
Thermotropic liquid crystals are also being explored as potential replacements for load-bearing titanium-based implants used to repair fractures in the long bones of arms and legs. Liquid crystalline polymers are being designed that can withstand high stresses as well as being osteoconductive (so they encourage new bone growth), biocompatible (so the body won’t reject them) and biodegradable (so a second operation isn’t required to remove them), explains materials chemist John Goodby at the University of York, UK.
Biodegradable polymers have been used in medicine since dissolvable stitches were introduced the 1960s. In recent years, they have entered orthopaedic surgeons’ toolboxes: in plates, screws, pins and meshes for bone fracture repair. So far, however, a suitable polymer has not been found to replace titanium-based implants that support fractured load-bearing bones.
As well as circumventing the need for a second operation, dissolving implants offer another significant advantage over the status quo. When a titanium-based implant is removed, bones can re-fracture owing to the cells becoming ‘lazy’ while the titanium has been taking the body’s weight. Polymers can potentially be designed to slowly dissolve, meaning the load is gradually transferred to the healing bone.
However it is proving difficult to design a material that is both strong enough to support these long bones while also dissolving at a slow enough rate. ‘It’s tricky stuff to get the material just right and having the right properties, both biological and physical,’ says Goodby. Liquid crystals might overcome this conundrum, specifically aromatic main-chain liquid-crystalline polyesters.
These polymers are made by incorporating rigid aromatic units into the backbone of normally flexible polymers. The rigid units cause the polymer to orient itself like a liquid crystal. The polyester analogues are well documented for their high strength, but are typically insoluble. Tweaking the polyester backbone might allow the polymers to dissolve in the body at a rate that suits the recovery time of these long bones.
Goodby’s group, alongside researchers at York-based medical technology company Smith & Nephew, are one of the teams who have been working on a suitable material. They have prepared a series of liquid crystal polyesters with various diacids inserted in their backbone, and tested their strength, biocompatibility and temperature sensitivity with some positive results. ‘There is a lot of work to go in this area before these become a real practicality however,’ says Goodby.
Pharmaceutical challenges
But while some liquid crystals could potentially find biotech applications, others are a source of frustration for the pharmaceutical industry. Some small, flat molecules with aromatic cores, when placed in solution (at certain concentrations), spontaneously stack into columns. These are a class of lyotropic liquid crystals called chromonics. ‘We come across them quite a lot in the pharmaceutical industry,’ explains John Jones, a research scientist based at Bristol-Myers Squibb’s R&D facility in Moreton, UK. ‘A lot of our drug molecules are aromatic, they can be planar, and they can have chemical properties that result in them forming chromonic liquid crystals.’
The challenge for the pharmaceutical industry is that a lack of fundamental knowledge about how and why chromonic liquid crystals form means it’s not possible to predict if or when they will appear. A viscous gel might suddenly form during an aqueous wash in the discovery stage, or the liquid crystals might first appear during dissolution testing (to predict in vivo drug release profiles). Sometimes this ability to form liquid crystalline phases is present in the final drug, for example in the anti-asthmatic drug Intal.
‘From a pharma point of view [this is] something we need to understand a lot better,’ says Jones. ‘If something unforeseen like this happens, that we can’t predict or control, then it becomes a challenge.’ Jones is currently involved in a UK-wide collaboration aiming to improve the fundamental understanding of these types of liquid crystals. ‘Once you crack the relationship between the chemical structure and the liquid crystal structure then you can control your formulations,’ explains Gordon Tiddy, one of the project’s collaborators at the University of Manchester. ‘You can put in salts or other additives to modify the liquid crystal behaviour.’ Chromonic liquid crystals usually make it more difficult to bring a drug to market because it is harder to control the release rate, he adds. The project has involved modelling, synthesis and characterisation of various liquid crystals of this type. But as yet we still don’t really understand these materials, says Tiddy.
Liquid crystal lipids
John Seddon at Imperial College London, UK, is another chemist working to improve our fundamental understanding of how lyotropic liquid crystals form. ‘The main thrust of our research at the moment is on lipids,’ he says, ‘both ones extracted from cells and ones that are synthetically produced to be similar to those extracted from cells.’ An improved understanding of the physical properties that control the self-assembly of these lipids in water into liquid crystalline structures should help potential applications such as drug delivery and gene therapy come to fruition, Seddon adds.
These resulting structures have varying degrees of complexity. ‘Some of the structures are quite simple, like bilayer membranes, while some of them are more complicated and have 2D or 3D periodicity in them,’ he says. ‘We’re interested in understanding what controls the structure and the stability of these complex structures.’ His team uses a battery of physical chemistry techniques – including x-ray diffraction, optical microscopy, electron microscopy, calorimetric methods and solid-state NMR – to probe these self-assembly processes.
Of these it is time resolved x-ray diffraction that is the most useful technique, because the self assembly of lyotropic liquid crystals occurs so quickly – taking just a few seconds or even milliseconds. To study the faster transitions, Seddon’s team uses synchrotron x-rays at either the UK’s Diamond Light Source or the European Synchrotron Radiation Facility in France. These higher intensity x-rays allow them to obtain up to five diffraction patterns per second.
Seddon says that some of the most exciting work his team is doing now is observing the assembly of 3D-ordered sponge-like cubic phases of lipid-based liquid crystals. These phases ‘have turned out to be very useful in applications such as crystallisation of membrane proteins, which led to a Nobel prize in 2012,’ he says. ‘It’s becoming clear that these complex cubic phases are involved in diverse structures within cells. So for example, the colours of butterfly wings are actually not due to pigment in the wing, but they’re due to a physical optical effect: a photonic crystal. The photonic crystal is derived from one of these cubic phases forming within the wing cells themselves.’
With much left to understand, the potential biological applications of liquid crystals are seemingly endless. ‘In the future, my guess is that the biggest developments in liquid crystals will be in biotech and pharmaceuticals,’ predicts York’s Goodby. ‘The moment you link pharmaceutical activity to liquid crystallinity, which has not been done yet, all the pharmaceutical companies will switch that way.’
Nina Notman is a science writer based in Salisbury, UK
References
- C R Safinya et al, New J. Chem., 2014, 38, 5164 (DOI: 10.1039/c4nj01314j)
- Y Fukushi et al, J. Mater. Chem.B, 2014, 2, 1335 (DOI: 10.1039/c3tb21736a)
- H Montes de Oca et al, Biomaterials, 2010, 31, 7599 (DOI: 10.1016/j.biomaterials.2010.07.006)
- A Akinshina et al, Soft Matter, 2015, 11, 680 (DOI: 10.1039/c4sm02275k)
- Faraday Discuss., 2013, Lipids and membrane biophysics, RSC Publishing












No comments yet