Our gut microbiome has been linked to conditions such as Parkinson’s and Alzheimer’s. Anthony King reports on the connections
You feel tension in the pit of your stomach as you begin your big public talk. You get butterflies as you wait for exam results. A mentor tells you to trust your gut feelings on a career decision. It’s no wonder ancient thinkers viewed the gut as the seat of emotions, or that a medieval physician even proposed that perception and our soul resided in our digestive organs. ‘Back in history we used to think the gut ruled everything,’ says microbiologist Brett Finlay at the University of British Columbia in Canada, ‘which I find amusing, now that we are getting back to that.’
Results from patients and animal models implicate gut microbes in Parkinson’s and Alzheimer’s diseases. Most Parkinson’s patients have a distinctive microbial signature in their gut. Put this microbiome into a Parkinson’s-like mouse and its condition deteriorates. Knock out the microbiome of Alzheimer’s or multiple sclerosis (MS) mice and their disease gets better, observes Howard Weiner, neurologist at Harvard: ‘That suggests the microbiome is participating in the disease.’
It is not only diseases. Mice without gut bacteria are more anxious. A 2011 study made timid mice into explorers by swapping their gut microbes with brave mice. ‘We’ve now shown unequivocally that the microbiome can affect a variety of different functions and activities of the brain,’ says Weiner. He believes such investigations can help us treat patients with Alzheimer’s and multiple sclerosis. Perhaps it’s not so surprising when the gut is home to 100 trillion bacteria, according to estimates, around 10 times the number of cells in the human body and they have evolved with us.
Gut–brain highway
The initial evidence for microbes influencing our brain came from animal studies. ‘When you go from a germ-free to a colonised mouse, the chemistry is 70% different in the gut but also 20% different in the brain,’ says Pieter Dorrestein, a chemist at University of California, San Diego, in the US. Heretical ideas have become mainstream. ‘If I said 10 years ago that Parkinson’s, Alzheimer’s, even obesity is influenced by microbes, I’d be getting weird stares’ says Finlay. ‘The data has piled up. It is probably the biggest change in medicine.’
How gut microbes hold such sway lies in three major gut-to-brain connections. First, the gut is chock full of nerves wired to the brain; second, 70 to 80% of our immune cells are in our gut and some commute to the brain; third, microbes generate or control a myriad of biologically active molecules, including hormones and neurotransmitters. The three paths from gut to brain also intersect with one another.
One major hallmark of Parkinson’s is that the microbes that are anti-inflammatory are much decreased
One class of bioactive compounds is the short-chained fatty acids (SCFAs), compounds produced when some bacteria ferment fibre in the lower colon. Fibre from fruit and vegetables gets converted into short molecules – two to four carbons long – such as butyrate, propionate and acetate. Butyrate is known to act as an anti-inflammatory agent. Some Clostridium bacteria produce a lot of butyrate and have a long history as probiotics.
Butyrate interacts with proteins that control which genes are turned on in immune cells. These SCFAs ‘are very short molecules, but they’re fundamental in modulating our immune system’, says Sergio Baranzini, a neuroscientist at the University of California, San Francisco, in the US. ‘Adding butyrate or propionate to the diet or even to immune cells in a culture modulates the action of T cells,’ a type of white blood cell trained to kill suspicious cells, by mustering regulatory T cells, their controllers.
Crucially, our diet influences microbial compounds. Processed food is too easily absorbed, for instance, starving the microbiome. ‘A diet diverse and rich in fibres promotes a more diverse community of bacteria, and more short-chained fatty acids will be produced,’ says Baranzini. ‘If there’s only fast food, then there is little for the bacteria in our intestines to process and to get these short-chained fatty acids.’
This spotlights a role for diet in brain diseases. ‘One major hallmark of Parkinson’s is that the microbes that are anti-inflammatory, which create short-chained fatty acids, are much decreased,’ says Finlay. A recent study of 490 people with Parkinson’s and 234 healthy people identified 85 species of gut microorganisms linked to Parkinson’s, some increased, some decreased, with a reduction in anti-inflammatory agents.
The Parkinson’s gut connection
Parkinson’s is a progressive disease with symptoms of tremor, difficulty walking and rigidity, mostly diagnosed in those 60 and older; there is a loss of dopaminergic neurons and abnormal knots of alpha-synuclein (Lewy bodies) in the brain. However, there’s growing evidence that Parkinson’s may kick off in the gut. Patients frequently experience gut symptoms, notably constipation, a decade before a diagnosis. One study detected Lewy bodies in the gastrointestinal (GI) tract of patients up to 20 years before a diagnosis.
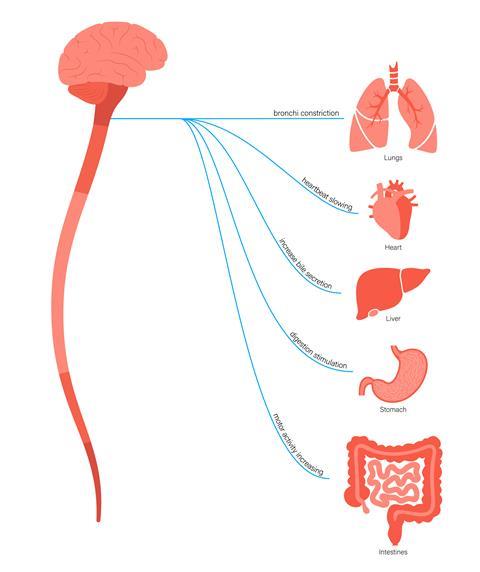
The vagus nerve connects your intestines to your brain. ‘The gut has its own nervous system, sometimes referred to as the second brain, and has more nerves than the entire spinal cord,’ says John Cryan, pharmacologist at University College Cork in Ireland. It is hypothesised that alpha-synuclein could be misfolded in or close to the gut, and then transmitted through the nervous system to the brain, similar to prion-like Creutzfeldt–Jacob and Alzheimer’s diseases. Intriguingly, in a Danish study, those with their vagal nerve surgically cut were at lower risk of Parkinson’s, perhaps because this serves as a transit route for misfolded protein going from gut to brain.
Parkinson’s patients show disturbances in their gut menagerie, with Helicobacter pylori gastric infection more likely present, a decrease in Faecalibacterium, and more Lactobacillus. Leakiness of the gut might also be a player. A healthy community of gut microbes is diverse, suppresses inflammation and is less likely to misfold alpha-synuclein, says Finlay, but this shifts with age. ‘As you get older, your gut gets more permeable, inflammatory molecules seep into the body to trigger inflammation, and misfolded alpha-synuclein in the gut works its way up the vagus nerve into the brain.’
How the misfolding starts is something that has intrigued biologist Paul Wilmes at the University of Luxemburg. His research has focused on microbes that might mess with alpha-synuclein, and others that may help sneak it into gut neurons. His lab noticed an enrichment of bacteria in Parkinson’s patients that feed off the mucus lining our gut – including Akkermansia muciniphila and Bacteroides. Switching mice to a low fibre diet encouraged these same bacteria. ‘This in turn led to foraging of the mucus layer and its thinning, leading those mice to become more susceptible to microbial toxins that can trigger protein misfolding,’ says Wilmes. Rodents enjoying fibre-rich food, however, had healthier communities of fibre-eating microbes that outcompeted the mucus grazers, improving the gut wall barrier – and made more SCFAs to boot.
Wilmes and his colleagues then zoomed in on curli, a protein made by E. coli and Salmonella species. This sticky protein helps such enterobacteria form biofilms, but it is suspected of clumping human proteins together too. A US group reported in 2016that rats exposed to curli-producing bacteria had more clumps of misfolded alpha-synuclein in gut and brain neurons. Wilmes found that a low-fibre diet in the presence of curli exacerbated disease in a mouse model of Parkinson’s disease. ‘Molecules such as curli, in combination with loss of barrier function, could trigger disease,’ says Wilmes. He is now working with Parkinson’s patients who are first having a colon cleanse and then further purging their microbiome by severely restricting calorie intake. The next step will be to put them on a Mediterranean diet, high in fibres and oils that feed beneficial microbes, particularly those that make SCFAs. This could restore the gut mucosal protection and reduce inflammation.
Over a dozen studies show the microbiome of Parkinson’s patients as distinct. ‘We can tell with 80 to 90% accuracy whether you have Parkinson’s based on your faeces,’ says Finlay. Unfortunately, he suspects that once misfolded protein is extinguishing brain cells, it is too late to stop it. One question now is whether it is possible to flag an unhealthy microbiome that is putting someone at risk of Parkinson’s or Alzheimer’s years before symptoms, prompting an intervention that stops the disease progressing.
Alzheimer’s and gut bugs
Alzheimer’s is marked by amyloid plaques and tau tangles, triggering cell death and brain shrinkage. Microbes are in the crosshairs here too. In a recent study, memory impairment in people with Alzheimer’s was transferred to animals using faecal transplants. The young rats suffered cognitive symptoms and systemic and gut inflammation. There was a fall in some bacteria (Firmicutes) and rise in others (Bacteroidetes), matching what is seen in patient groups. It confirmed a causal role for the gut microbiota in Alzheimer’s disease, according to the study scientists.
Laura Cox, a microbiologist at Harvard Medical School in Massachusetts, US, was hooked by an early career discovery connecting the microbiome, metabolism and Alzheimer’s. Meanwhile, her colleague Marissa Schafer, now at the Mayo Clinic in the US state of Minnesota, discovered that calorie restriction could prevent amyloid plaques in female lab mice. Together they found profound changes in gut microbes during ageing in mice, linked to plaques.
When we give Bacteroides to mice, we see increased plaques in their brain
The microbiome is known to become a little less stable after 60 years of age, sometimes allowing a few microbes to dominate. A 2021 US studyshowed that people over 85 years who had a relatively high levels of Bacteroides and low gut microbiome uniqueness were more likely to die within four years.
Cox reportedthat Bacteroides fragilis in the gut of mice was associated with increased plaques in their brain. ‘Multiple studies have found that Bacteroides is elevated in Alzheimer’s disease,’ says Cox. ‘When we give Bacteroides to mice, we see increased plaques in their brain’. She thinks it could be secreting something into the bloodstream or tweaking the immune system.
One initial hypothesis was that gut inflammation was the culprit, but their subsequent research sketched a more complex picture. ‘We found that a long, slow alteration in the gut microbiota led to immune senescence in the brain, not inflammation, says Cox.
In essence, a pro-inflammatory gut was akin to a constant alarm, and the immune system adjusted by tuning down its response and hit a snooze button. Specialist microglia cells that roam the brain normally chew up plaques and repair tissue, but they begin to slack off. ‘We think there could be a slow accumulation of plaques over 20 years, giving you a lot more amyloid beta in the brain,’ says Cox.
Yet the variability of Bacteroides means their link to Alzheimer’s is no slam dunk. In irritable bowel syndrome, only Bacteroides vulgatus strains that make a specific protease caused symptoms;while strains that lack the protease don’t contribute to disease. Similarly, there might be good and bad Bacteroides in brain diseases. ‘We’ve now looking for a unique strain of Bacteroides in our Alzheimer’s research,’ says Cox.
This hits on a crucial point. While researchers can recognise an unhealthy microbiome, a healthy one is harder to define and much more variable. And having a species name like Bacteroides fragilis doesn’t tell you about what compounds its churning out. ‘Instead of looking at what microbes you have, you need to look at the metabolic pathways they encode. You might need 10,000 genes expressed in the gut, and it doesn’t matter which microbes do it,’ says Finlay.
This means that there is plenty of redundancy and a complex community is more robust. Moreover, one strain of a common microbe might be beneficial or harmful, depending on the context and the genes expressed. ‘A problem in the field is that it is dominated by microbiologists who want to put microbial names on everything, but we should be looking at their function,’ says Finlay. There is potential good news too. If there is a bad B. fragilis, there could be a good B. fragilis to replace its evil doppelganger via a therapeutic infusion.
There are times when an acute infection and high levels of inflammation could contribute to Alzheimer’s
Meanwhile, Cox is seeking a small molecule that could wake up the immune system, switching on the clearance of plaques. This could potentially be mediated through the gut, where most immune cells get schooled in the microbial world. Weiner, however, has followed a different path. He launched a vaccine made up of bacterial parts, Protollin, for injecting into the nose of people and stimulating the immune system to rouse microglia. Over a decade ago, it was reportedto clear brain amyloid in mouse models. Now it is in human trials.
Others say infectious agents trigger Alzheimer’s more directly. ‘Amyloid-beta is an antimicrobial peptide that is produced in the brain to protect against microbes,’ says Rudy Tanzi, a neuroscientist at Harvard Medical School. He believes that in some people, an infection ignites a match and a wildfire of inflammation takes off and leads to an over-accumulation of otherwise protective plaques and tangles. Various infectious agentshave been implicated in Alzheimer’s, including herpes virus, Epstein–Barr virus, Helicobacteri pylori, and the yeast Candida albicans. Tanzi is examining postmortem tissue from Alzheimer’s patients to investigate such links. Although Cox argues that a jaded immune system has a role in the disease, she agrees that ‘There are times when an acute infection and high levels of inflammation could contribute to Alzheimer’s.’
Microbial compounds on the brain
The trillions of bacteria in our gut can orchestrate thousands more chemical reactions than human cells. Standout molecules include the bile acids, which are cholesterol metabolites made in the liver that solubilise dietary fats and help with absorption or secretion through the gut. ‘Microbes conjugate them to various molecules, including neurotransmitters, and make secondary bile acids,’ says Sarkis Mazmanian, a microbiologist at Caltech in the US. Bacteria also tweak them through hydrolysis, oxidation, defsulfation and esterification, a recent review noted.
Dorrestein says around 20,000 types are now known, up from less than a thousand a year ago. Moreover, though the blood brain barrier is often conceptualised as an impermeable wall, there are portals for microbial compounds. ‘There are bile acid receptors and transporters in the brain,’ affirms Dorrestein. One recent study looked at over 2000 postmortem brains and found altered cholesterol metabolism and bile acid synthesis in brain tissue and hints of bile acids coming from gut microbes. ‘Bile acid profiles are different in Parkinson’s or Alzheimer’s [patients] compared to matched controls,’ says Mazmanian, another tantalising glimpse of their contribution. Dorrestein adds: ‘They probably control lots of brain functions, sometimes mediated via inflammatory processes.’
The brain is complex, the immune system is complex and now we are adding the microbiome
Sulfated small molecules are derivatives of amino acids like tyrosine and tryptophan and seem to be made solely by microbes. Some gain access to and dock to receptors on brain cells. Altered phenolic metabolite levels have been reportedin autism, including 4-ethylphenyl sulfate. This compound was linked to anxiety and autism-like behaviour in lab mice in 2013. ‘We are talking about a molecule that we do not make, that comes from microbes, but gets into the brain and affects the activity of brain cells and behaviour,’ says Mazmanian.
This underpinned a recent trial in New Zealand and Australia. Mazmanian and his colleagues gave 30 teenagers with autism a spherical carbon adsorbent to catch phenolic compounds produced by gut bacteria, and in 2022 reported improvements in anxiety and GI health after eight weeks.
‘Gut microbes are like a factory, producing weird and wonderful chemicals,’ says Cryan. ‘The quality of the workers matters, meaning bacteria, and the quality of the materials, meaning diet.’ As part of the Alzheimer’s Gut Microbiome Project Consortium, Dorrestein and his colleagues recently showedthat a high-fat modified Mediterranean diet for six weeks can benefit adults with mild memory problems. In a preprint, the consortium is reporting that this diet righted some metabolic disturbances linked to Alzheimer’s and reduced systemic inflammation.
For now, dietary interventions and probiotics are often tested, but ultimately the field wants small molecules to trial in diseases such as Alzheimer’s and Parkinson’s. It won’t be easy. ‘The brain is complex, the immune system is complex and now we are adding the microbiome,’ says Baranzini. ‘This is a field here for the long run. There will be sporadic progress, but it will take time.’
Anthony King is a science writer based in Dublin, Ireland

















No comments yet