How organocatalysis won the Nobel prize
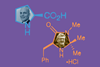
Jamie Durrani tells the story of how two young upstarts, Ben List and David MacMillan, created a whole new field of catalysis
On Wednesday 6 October, Ben List, a chemist from the Max Planck Institute in Mülheim and Princeton University’s David MacMillan were awarded the Nobel prize ‘for the development of asymmetric organocatalysis’. Such has been the impact of their work, many people close to them had been expecting the call from Sweden to come for several years.
Often described as the ‘third pillar of asymmetric catalysis’, organocatalysis offers synthetic chemists an alternative way of making chiral molecules that does not rely on transition metals or enzymes.
The story of List and MacMillan’s achievement begins in the late 1990s in California, when both researchers started off their independent academic careers, List at the the Scripps Research Institute in La Jolla and MacMillan at the University of California, Berkeley.