As multiple novel male contraception compounds enter clinical trials, is family planning about to undergo a second revolution? James Mitchell Crow reports
-
Emerging male contraceptives: Multiple novel male contraceptive compounds, both hormonal and non-hormonal, are entering clinical trials. These include small molecule inhibitors like sAC inhibitors, which offer the potential for on-demand contraception.
-
High demand and interest: Research indicates strong interest in new male contraceptives from both men and their partners. This interest has increased significantly in the US following the 2022 Supreme Court decision on abortion rights.
-
Challenges and innovations: Developing male contraceptives involves overcoming challenges such as ensuring minimal side effects and achieving effective, reversible contraception. Innovations like structure-assisted drug design and selective inhibitors are key to advancing these efforts.
-
Future prospects: The first generation of male contraceptives is expected to be hormonal, followed by non-hormonal and on-demand options. Regulatory and pharmaceutical company engagement will be crucial for bringing these products to market.
This summary was generated by AI and checked by a human editor
When the oral contraceptive pill was introduced in 1960, women who could access it gained a newfound agency to plan parenthood, creating cascading societal change. Since that time, their contraceptive options have expanded to include multiple forms of hormonal pills and patches, implants, and hormonal and non-hormonal intrauterine devices.
A recent count put the number of reliable, reversible contraceptive options for women at 14, with more in the pipeline. For example, a progestin-only injectable now in clinical trials could soon offer women a long-acting and reversible contraceptive with improved safety and fewer side effects than today’s options.
But despite these advances, unintended pregnancy remains common. Across low- and middle-income countries, the rate of unplanned pregnancy is 49%. Even in the US and the UK, with high access to modern contraceptives, approximately 45% of pregnancies are unintended. Although not all unintended pregnancies are unwanted, more than half end in abortion.
A broader suite of contraceptive options is clearly required – not least for the half of the population that still has very limited choice. More than six decades after the introduction of the female birth control pill, the number of contraceptive options for men remains stuck at two: the essentially non-reversible vasectomy, or the male condom. With a typical failure rate of 13%, male condoms are far less reliable than the reversible contraceptive options available to women.
Interest in new male contraceptives – from men and from their partners – is high. A recent multi-country assessment of male contraceptive demand found that 61% of men would be interested to try a novel male contraceptive. In the US, men’s interest in novel male contraceptives jumped from 39% to 49% in the wake of the 2022 US Supreme Court’s decision to overturn the constitutional right to abortion.
I don’t think there’s ever been this many different product forms for male contraception in clinical trials
The research also found that female partners’ interest, and trust, in male contraceptive use was high. For many women, hormonal birth control methods have side effects. A male pill would enable the burden as well as the opportunity of contraception to be more equitably shared.
Soon, questions over novel male contraceptive uptake may no longer be hypothetical. ‘From the medicinal chemistry point of view, I don’t see male contraceptive development as any different from the female contraceptives that are in the market right now – it is a completely solvable problem,’ says Min Lee, a chemist in the contraceptive development program at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in the US.
After decades of stalled progress, dedicated research funding programmes from the NICHD and from male contraception advocacy groups have reinvigorated the field. Two non-hormonal and four hormonal male contraceptives have recently begun human clinical trials, deploying diverse chemistries to prevent unintended pregnancy. At least a dozen other options are close behind, in the development to pre-clinical phase of research.
‘We’ve recently seen pretty much the entire field of male contraception take a big step forward,’ says Logan Nickels, chief research officer at the Male Contraceptive Initiative (MCI), a US-based advocacy organisation turned research funder. ‘I don’t think there’s ever been this many different product forms for male contraception in clinical trials.’
Pregnant pauses
The stop–start story of male contraceptive development, and the recent surge in activity, is typified by the development of small molecule inhibitors of an enzyme called soluble adenylyl cyclase (sAC). This contraceptive target was a chance discovery by Jochen Buck and Lonny Levin at Weill Cornell Medicine in New York, US.
The pair began working together during the 1990s, when Levin was puzzling out some of the fundamental biochemistry of cyclic AMP, a common second messenger in mammalian cells. Following up on a literature lead about a potential cAMP-producing enzyme in the testes, Levin and Buck teamed up to track down and purify and sequence this enzyme.
The enzyme they identified, sAC, turned out to be closely related to cAMP enzymes in cyanobacteria, some of the most ancient forms of life. For a protein to be conserved for so long suggested important function. ‘We dropped everything to study this together,’ Levin says. The work remained fundamental research – but the more the team studied sAC, the more the enzyme’s potential as a novel contraceptive target became apparent.
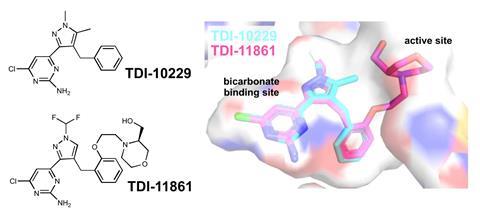
First, in 2001, the team showed that sAC was a key player in a puzzling piece of sperm behaviour involving bicarbonate. Throughout the body, the bicarbonate concentration in extracellular fluid is typically 25 millimoles per litre. But in the cauda epididymis of the testes, where sperm are stored at the end of the approximately 10-week sperm production process, the extracellular fluid bicarbonate concentration is below 5mM.
‘When a man ejaculates, a tiny pellet of sperm is mixed with semen fluid, which has the regular 25mM bicarbonate,’ says Buck. ‘That’s the signal for sperm to start to move.’ The bicarbonate boost was known to increase cAMP production, triggering swimming, but the molecular mechanism was unknown. Levin and Buck showed that their sAC enzyme was directly regulated by bicarbonate. ‘We now have a crystal structure that shows exactly where the bicarbonate binds,’ Levin says.
Not long after, the team produced mice with the gene for producing sAC removed. Females were unaffected and males also grew up normally, except that they were infertile. ‘All these things suggested in theory that we had a contraceptive target,’ Levin says. ‘But now here comes the problem.’ In discussions with pharmaceutical companies, the team pointed out that sAC is not found solely in the testes – suggesting potential for off-target effects.
‘If you think about drugs that treat a health condition, you’re willing to put up with certain amounts of side effects if they’re less than the ramifications of disease,’ says Levin. ‘Male contraception isn’t like that.’
Male contraceptives would be taken by healthy men, for whom – unlike for women – pregnancy and childbirth carry no personal health risk. ‘So what side effect profile is acceptable? Pharma said that the risk profile should be zero,’ Levin says. Given that sAC was not sperm cell specific, the firms they worked with ‘shut down the programme and went home’.
On demand
Resuming their fundamental work, Levin and Buck started developing selective sAC inhibitors to study its biology. Over a decade later, in 2019, two key developments changed the game again. First, other researchers reported a family of infertile but otherwise fit and healthy males with mutations in their sAC gene.
‘This showed that a man can go through life without sAC, and have essentially nothing wrong with them except for being infertile,’ says Buck. ‘That gave us a bit of confidence that this target is OK.’
At that time, the team was also testing its latest selective sAC inhibitors in mice, when a postdoc decided to check the effect on the mouse’s sperm once the main experiment was done. An hour after the inhibitor injection, the mouse’s sperm was immobile. A day later, the same animal’s sperm had returned to normal. Repeat experiments confirmed the observation.
‘That’s when we had the epiphany,’ Buck says. ‘This was not just a male contraceptive, it was an on-demand contraceptive, unlike anything else available.’ Uniquely, a sAC inhibitor could potentially be taken as a single dose shortly before sex, rather than a pill that must be taken every day. A contraceptive only taken as required could also lower the possibility of side effects compared to a daily pill.
An on-demand male contraceptive would be a paradigm-changing technology
The third factor was that, although major pharmaceutical companies remained on the sidelines, funding was now available through the MCI. ‘MCI was founded in 2014 as an advocacy organisation, trying to increase public funding for male contraceptives and boost public support,’ Nickels says. But in 2017, MCI attracted a major anonymous donor. ‘So, we became a grant-making institution as well,’ he says. The sAC project is one recipient of the approximately $15 million (£11.8 million) in grants that MCI has made to date for non-hormonal reversible male contraception development.
Structure-assisted drug design has been key to the sAC project since that time. The team found that their initial compounds, which solely engaged the sAC’s bicarbonate binding site, would quickly fall off their target once the sperm was ejaculated into the drug-free female and the inhibitor concentration fell. A more recent derivative overcame this issue by incorporating a bigger side chain also designed to engage with the channel leading to the enzyme’s active site. This compound was shown to be an effective on-demand temporary contraceptive in mice.
The team established a spin-out, Sacyl Pharmaceuticals, to further fine-tune the molecular structure to improve its oral bioavailability and wider pharmacokinetics. Before the end of 2025, the aim is to have a clinical candidate to take into clinical trials.
Inhibiting sperm motility by this mechanism could also be developed as a female contraceptive. As well as its on-demand male pill work, the team is collaborating on a vaginal ring that would elute the sAC inhibitor, as well as an antiviral compound, in a USAID-funded programme for women in Africa.
‘An on-demand male contraceptive would be a paradigm-changing technology,’ says Nickels. ‘That they can target sperm function by delivering drug to women or men is really cool because it symbolises the equity that we want to bring into the contraceptive conversation – that male contraception is about providing more options to more people.’
Currently, sAC inhibitors are a little behind the leading pack in male contraceptive clinical development, says Levin. But the drug’s mode of action, to stop sperm swimming, means that already in a phase 1 trial, efficacy could start to be assessed from sperm samples. ‘And we simply check, does it swim or not?’ Buck says.
Calling medicinal chemists
One male contraceptive already in a second phase clinical trial is YCT-529, a non-hormonal compound designed to target retinoic acid receptor α (RARα). As with sAC inhibitors, the project has had a long gestation period. Lead medicinal chemist on the project, Gunda Georg from the University of Minnesota, US, started work on male contraceptive compounds almost 15 years ago, but RARα biology research goes much further back. ‘When I started, there was no medicinal chemist working on any of this,’ says Georg.
At that time, the NICHD team had recognised this problem and set out to address it. ‘When I joined NICHD 14 years ago, the research was all target discovery, biology and biochemistry,’ says Lee. ‘I saw immediately that if we wanted to move this to the next level, we had to broaden our scope to include chemistry.’
The NICHD offered a research contract to develop non-hormonal male contraceptives, stipulating that the principal investigator had to be a medicinal chemist. The aim was to start developing candidate molecules against some of the targets that biologists had identified. ‘I thought this is a very interesting topic where I could make a contribution,’ Georg says.
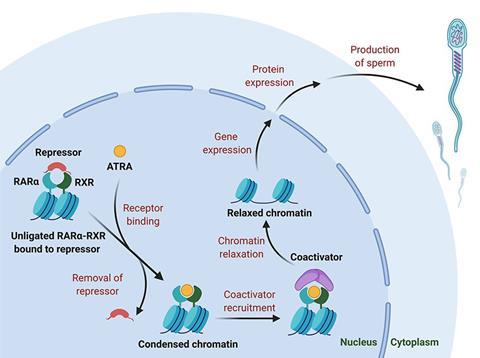
NICHD connected Georg with reproductive biologist Debra Wolgemuth at Columbia University, New York, who had been investigating RARα. As far back as the 1930s, researchers had found that male animals deprived of vitamin A (retinol) became infertile. Eventually, tracing the mechanism, the interaction between the active metabolite, all-trans retinoic acid (ATRA), and RARα was found to be essential for normal spermatogenesis. Mice lacking the gene for RARα had validated the receptor as a male contraceptive target, so Georg began developing small molecule antagonists that mimicked the structure of ATRA to fit the binding site on RARα, but incorporated bulky side groups to disrupt the receptor’s function.
As the body contains several RAR subtypes, strong selectivity was a prerequisite. Uniquely, RARα has a serine residue in the binding pocket where the other isoforms have an alanine. ‘If you can establish a hydrogen bond to that serine, you may get a selective compound,’ Georg says. ‘We focused on compounds that could potentially make that hydrogen bond.’
Among the multiple structural combinations that the team trialled was YCT-529, which featured a pyrrole ring in the key hydrogen bond position. The compound proved to be a highly selective RARα antagonist. It also had excellent pharmacokinetics and high oral bioavailability, Georg says. In mice, the compound was 99% effective and 100% reversible. In primates, the sperm count fell below fertile levels within two weeks of commencing the drug.
Georg is working with Your Choice Therapeutics to bring the compound through early-stage clinical trials. After successfully completing a human phase 1a drug safety trial in early 2024, in September 2024 the company began a phase 1a/2b study to further test safety and assess the compound’s ability to lower sperm count in men.
Playing tag
In Georg’s academic lab, research continues apace on selective inhibitors of another non-hormonal male contraceptive target, cyclin-dependent kinase 2 (CDK2). Longer term, her team is also targeting a sperm-specific ion channel called CatSper, which is required for male fertility. ‘A lot of people think CatSper is the best of all targets because it is exclusively expressed in the testes,’ Georg says. ‘But there are a lot of other ion channels in the body, and so we still have to deal with selectivity.’
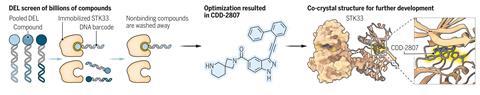
In other labs’ research, Georg adds, one of the most promising recent studies was conducted by Martin Matzuk at Baylor College of Medicine in Houston, US. Matzuk has developed DNA-encoded small molecule libraries that each contain 3.9 billion specific DNA-tagged compounds. He recently deployed these huge libraries to identify hits against another male fertility associated kinase, STK33.
‘When I did a CDK2 screen, we tested maybe a hundred thousand compounds,’ Georg says. ‘It’s just mindboggling that you can now screen billions of compounds,’ she says. From the hit that the screening generated, Matzuk’s team applied medicinal chemistry refinements, and demonstrated in mice that the compound safely induced a reversible male contraceptive effect. The study confirmed STK33 as a druggable male contraceptive target, and the next step is to develop analogues with oral bioavailability so that it could be formulated as a pill rather than an injection, Georg says.
As a drug discovery tool, you can’t compete with a library containing billions of compounds, Georg adds. ‘I’m now also using this DNA encoded library screen,’ she says.
On the pill
Also among the non-hormonal male contraceptives currently in early stage clinical trials is a hydrogel designed to reversibly plug the vas deferens, blocking sperm flow. But the most clinically advanced compounds of all – following the precedent set by female contraceptive development – are hormonal male contraceptives.
Sperm production depends upon a high concentration of testosterone in the testes, 40–100 times above that of testosterone levels in the blood. The process is regulated by the hypothalamus in the brain, which senses testosterone levels in the circulation and signals to the testes when more is required.
Male hormonal contraceptives are designed to subvert this system. When men are given extra testosterone or other androgen or progestin hormones, the hypothalamus perceives that levels are high and so shuts down testosterone production in the testes. Once testosterone concentrations in the testes drop below a threshold, sperm production ceases. But trials showed that simply shutting down testosterone production for male contraception caused significant side effects including to libido, erection, ejaculation and muscle mass.
The strategy around this problem has been to develop hormonal contraceptives that provide ‘hormonal addback’, avoiding side effects by topping up testosterone (or related androgens) levels in the bloodstream while keeping testosterone production in the testes shut down to halt sperm production. The hitch with developing this concept into a contraceptive pill has been that testosterone taken orally is cleared so rapidly that men would have to take multiple pills a day.
Chemists at the NICHD have pioneered one route around this problem. They have designed two compounds, dimethandrolone undecanoate (DMAU) and 11β-methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC), which are prodrugs of modified testosterones. The molecules feature cleavable ester or carbonate sidechains that improve oral bioavailability and slow clearance.
Once the side chain is cleaved, the active modified testosterones that are released bind to both androgen and progesterone receptors. DMAU and 11β-MNTDC can therefore be used as a single molecule that suppresses testosterone and sperm production while simultaneously providing hormone addback to counter side effects. ‘We think that the carbonate side chain linker is probably the better fit for a once-a-day pill,’ says Lee.
The gel might appeal to users who don’t want to take a pill
In early-stage clinical trials, both compounds were found to be safe and to suppress testosterone production in the testes. Longer trials to assess sperm production suppression are planned. Currently, however, NICHD is focusing its resources on an alternative solution to the oral testosterone rapid clearance problem. The organisation is leading the clinical testing of a contraceptive gel, rubbed into the shoulders once a day, which contains Nestorone (segesterone acetate) and testosterone.
In June 2024, the NICHD team announced interim results of an international phase 2b trial, showing that the gel does suppress sperm production. The trial continues to test the contraceptive’s effectiveness, safety, acceptability and reversibility.
‘From a chemical perspective as well as a contraceptive perspective, I think they’ve done a really great job of generating different product forms that get around testosterone’s bioavailability and oral issues,’ Nickels says. ‘The gel might appeal to users who don’t want to take a pill,’ he adds.
A rising tide
Hurdles remain – not least, drug regulator and pharmaceutical company engagement as these compounds reach large and costly final stage clinical trials. ‘We see big pharma at male contraceptive conferences, these are things they want to stay appraised of – but they are not at the table yet,’ Nickels says. ‘It’s risky because you have to generate a product that is very, very safe, and very, very efficacious – and so I think industry sees the financial risk of early product development.’
Adding further uncertainty, there’s little precedent for a phase 3 male contraceptive clinical trial, and so it remains unclear as to what drug regulators such as the US Food and Drug Administration (FDA) would want to see from such a trial to grant a male contraceptive approval.
The NES-T topical gel is likely to be the first male contraceptive candidate to enter phase 3. ‘The FDA doesn’t have an example to follow, so we and the FDA will be breaking new ground,’ says Lee. ‘There’s a lot of complexity and caution associated with getting the trial design right.’ Hopefully, within two or so years, the final stage clinical trial for the gel can start, he says.
If these issues can be overcome, Nickels sees male contraceptives as becoming available in a series of waves. ‘The first-generation male contraceptives are going to be the hormonal products, and the second-generation products are going to be the non-hormonal options and maybe the on-demands,’ he says.
A third generation could be options such as highly targeted CatSper inhibitors now being explored, Nickels adds. ‘There’s about 4 billion men on this planet, and I think each method is going to appeal to a subset of users,’ he says.
‘Since starting work in contraception, I’ve really come to appreciate the impacts it can have on the world,’ Nickels adds. ‘It affects people’s lives at their core, and can have demonstrated outcomes in terms of economics, education, general health and wellbeing. Preventing unintended pregnancies is a rising tide that can lift all boats.’
James Mitchell Crow is a science writer based in Melbourne, Australia


















No comments yet