Researchers working with automated systems are pushing the boundaries of what chemists can achieve in the lab, reports James Mitchell Crow
For any aspiring young chemist, the first few months in a university research lab can be challenging, a daunting learning curve. But for one bright new thing, recently arrived in Andy Cooper’s lab at the University of Liverpool, UK, the first 12 months proved especially exasperating. ‘It was hard,’ Cooper recalls. ‘About one year in, I honestly thought we would never do it – it seemed impossible.’
This particular new recruit to the Cooper lab turned up in a box. ‘We were working on photocatalysis to make solar fuels, looking at making hydrogen by water splitting,’ Cooper says. ‘It is such a big chemical space, we wanted to build a platform that could do it autonomously: make the materials, test them, then decide what to do next.’
The team enquired about commercial systems with the necessary capabilities, but the quotes that came back were prohibitive. ‘It was going to cost around £5 million,’ Cooper says. ‘We didn’t have £5 million. But the epiphany was we already had all the instruments to do all the component jobs. It was a case of stringing them together.’

Cooper briefly flirted with employing a person to be that link, working under the direction of the artificial intelligence (AI) algorithm tasked with exploring this reaction space. ‘But who would want that job? So then we thought, we’ll get a robot to move the things around the lab,’ he says. ‘The robot does the measurements, the algorithm tells it what to do next – that could be at 3am, and then at 3.01am it’s doing the next set. And that’s exactly what we did.’
Getting the robot chemist concept working was fraught with challenges. ‘It was just me and the student, and it took us quite a long time just to get the thing to move across the lab, let alone to do an experiment,’ Cooper says. Once they go the robot rolling, its 400kg bulk broke the floor.
‘But the first time it did a full two-day experiment over a weekend, produced real data, was amazing,’ Cooper says. In the robot’s first major project, it conducted 688 experiments over eight days, adjusting 10 variables, to discover a photocatalyst mixture six times more active than the initial formulations.
‘The most fun thing is, you don’t know what the thing’s going to discover,’ Cooper says. ‘You’re logging in at 2am to see what it has found. It is a qualitatively different way to do things. I think we have only got a glimpse so far of what is possible.’
Non-human skills
Cooper wasn’t new to chemistry automation, having bought his first Chemspeed high throughput robotic synthesis platform in 2002. ‘There is sometimes a bit of a perception that this whole automation area started four years ago or something. Synthesis robots have existed for a long time,’ he says. ‘They’re programmable but don’t make decisions.’
‘We have had automation for a very long time – the secret is to get the AI to drive it,’ agrees Alán Aspuru-Guzik, who develops self-driving labs at the University of Toronto in Canada (see box Stirred, not shaken below). ‘What’s cool here is the coming together of AI and robotics,’ he says.
With rapid recent advances in AI, it suddenly becomes possible to envisage systems in which a computer runs a reaction, characterises the products, analyses the result, then selects the next experiment to autonomously explore chemical space. A diverse array of robot chemists is now beginning to swing into action, carrying out chemistry experiments in an AI-driven feedback-controlled fashion.
If a human can do it, you can stick it in the workflow
‘If you look at what AI does well, it is almost exactly converse to what humans do well,’ Cooper says. ‘An AI can make decisions in 30-dimensional space, which a human can’t. Equally, seeing a cat is a cat and a dog is a dog is trivial for a human but a hard challenge for AI,’ he adds.
That means there are certain areas of chemistry are a better fit than others for setting AI-endowed chemistry robots to work, Cooper says. ‘They’re very well suited to what you might call formulation problems, where you have a large number of components interacting in very complex ways that are hard to understand,’ he says. ‘Finding non-intuitive solutions that would not have come out of a rational design process – that to me is probably the most interesting aspect of the whole thing,’ he says.
From the hardware point of view, Cooper’s use of a mobile robot to link together existing lab infrastructure into an automated system is just one approach. ‘I think the distinctive thing about what we have done is the sheer range of manipulations and measurements you can put together,’ Cooper says. ‘Basically, if a human can do it, you can stick it in the workflow,’ he says, although adding each new capability can be time-consuming and non-trivial.
The more steps in the workflow, the greater the potential for things to go wrong. ‘A 1% failure rate when you are doing 10 things, you suddenly find things are failing all the time,’ Cooper says. ‘You need to adopt an engineering mindset and think about redundancy, safe failure – things chemists don’t usually think about.’
Stirred, not shaken
Alán Aspuru-Guzik started his career as a computational chemist, working on discovery of new functional materials. But after getting frustrated by the time experimentalists were taking get around to synthesising these materials, he decided to develop lab automation instead.

Since joining the University of Toronto in 2018, Aspuru-Guzik has held a join professorship in the departments of chemistry and computer science. In the lab in the computer science department, which is working on the robotics side of the story, one focus is developing robotic arms with a computer vision system able to recognise, pick up and manipulate glassware. ‘We are already able to do basic experiments,’ Aspuru-Guzik says.
With a ‘blind’ robot chemist – such as Andy Cooper’s system – the robot must be pre-programmed with the coordinates of all the vials and instruments it needs to conduct its experiment. That’s time-consuming, Aspuru-Guzik says. ‘Our goal, which I think is more realistic, is to be able to enter a disorganised area and just start to work. You can just place objects on a table, and the machine can recognise what is there, start to move things around.’
The challenge with glassware is that it is transparent, which can confuse AI vision systems. ‘Until last year, we held the record for transparent object recognition, with a system that combined a camera and depth sensor,’ Aspuru-Guzik says. After being briefly overtaken by another group, his team reclaimed the record using a system that takes multiple images of the object from different angles. ‘It is how we do it instinctively as humans, we look at objects from different angles,’ he says. The system can now recognise transparent objects such as flasks containing solvents, he says.
To road-test the technology, Aspuru-Guzik is creating an AI robot for mixing cocktails. ‘If we run out of tequila, the robot can decide what to try instead, whether something similar like mescal, or something quite different.’ It’s the prelude to a system that can make smart real-world decisions in the lab.
Go with the flow
Unlike Cooper’s rolling robot, most AI-driven robot chemists do not roam the lab. The more typical set up is a computer controlled stationary system including pumps, reactors and in-line analytics.
‘In my lab we have places that look like a normal organic lab, now upskilled with particular pieces of equipment, and then we have spaces for robots that people don’t go in,’ says Jason Hein, whose research at the University of British Columbia in Canada is focused on automated reaction analytics technology. ‘But when I say robot, it is not R2D2. It is an assembly of electronic equipment that can be programmed to do actions such as liquid handling, stirring, cooling.’
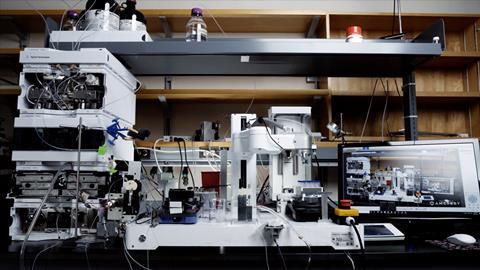
Like Cooper, Hein first looked to machine assistants when faced with a particular problem in the lab. ‘When I started my lab at University of California Merced in 2011, I was one of the founding faculty members to start the department of chemistry,’ he recalls. But Hein’s research, focused on detailed analysis of reaction mechanism, required some relatively expert skillsets that rookie students don’t come in with. ‘You had to do kinetic analysis, which was both tedious and exacting and precise, and they were not something you could do with day zero graduate students and undergrads. We pivoted very quickly to build tools that rapidly upskill people.’
Over the years, Hein’s lab machines became increasingly sophisticated. ‘To answer bigger questions, the tools had to get better – which led to bigger questions, pushing this cycle,’ Hein says. ‘We’ve built to the point – linking up with Alán Aspuru-Guzik and that AI community – that instead of clockwork programming we can now embed inquiry and learning into these tools.’
Removing the research barrier that requires students to master esoteric manual lab skills by developing tools that can perform these tasks faster, more consistently and more reliably, remains a core driver of Hein’s work. ‘In neurology, there’s an incredibly important measurement for neuronal cell diffusion called the Patch clamp technique,’ Hein says. ‘People that use it describe grad students as having “hands”, and a defining characteristic of succeeding or failing your PhD is, can you pull a glass pipette the right way. That’s not why you are there to be a neurobiologist, or a chemist.’
Increasingly, the second benefit of the technology is the both the scale and scope of the kind of questions Hein’s team can tackle. ‘When I started in this field, you’d find a reaction that was maybe two to three starting materials, maybe one by-product, two or three products,’ Hein says. ‘My grad student is looking at a system of 40 to 50 interconverting materials, and we can really model the true nightmare going on inside that flask. Normally for this kind of reaction, nobody would ever entertain taking on the kind of analysis we are doing.’
Hein’s students do emerge with practical lab skills – they are just different ones to those a typical physical organic chemist would have acquired. ‘The flipside is, we spend just as much of our time in the lab keeping the stuff running,’ Hein says. ‘I just had a graduate student finish, and her thesis quote, very apropos, is “The obstacle is the path”. It’s a different sort of skillset.’ One very much in demand, he notes.
‘Almost every graduate student has gone immediately into an industrial position,’ Hein says. ‘They can speak engineer, they can speak robot, they can speak computer science, they can speak chemistry. They’re this nexus for so many different fields to help them knit together.’
Speeding up discovery
Chemical engineer Milad Abolhasani spent almost a decade developing microfluidics and flow chemistry platforms for high throughput pharmaceuticals and advanced materials research. In 2016, when he joined North Carolina State University in the US he decided it was time for a new tack. ‘When you are looking for new catalysts, new materials, the problem space is just enormous,’ Abolhasani says. ‘With high throughput screening we were barely scratching the surface.’
Why do we want to have a self-driving lab? To accelerate our search through the chemical space
Take a six-parameter reaction system, for example, where you want to look at 10 different levels for each parameter. ‘That’s a million experimental conditions to have to run,’ Abolhasani says. ‘Even with high throughput screening, that’s going to take a lot of time and resources.’ AI and machine learning increasingly seemed to offer the possibility to explore the space more efficiently. ‘So my group started building hardware that fit AI machine learning.’
Microfluidic flow chemistry setups are ideally suited to this kind of AI-guided high-speed mapping of broad chemical landscapes, such as to identify novel catalysts with landmark performance, Abolhasani argues. ‘Why do we want to have a self-driving lab? To accelerate our search through the chemical space. So our set-up should also be very optimised,’ he says. The team developed a set-up to run experiments on microscale, using an inert fluid that separates the reagents from the reactor walls to remove the need for washing steps between runs. Running reactions in flow at this scale also increases the rate of mixing and heating, accelerating reaction kinetics. ‘We have done multiple examples in our lab for nanomaterial synthesis, showing we can reduce the total reaction time from few hours in batch to few minutes in a flow reactor.’ Photochemical reactions are similarly sped up, he says. ‘So within 24 hours you can do hundreds of reactions instead of a few you get to do in batch, and you end up consuming 3–10 orders of magnitude less chemicals.’
Getting this AI-driven setup built – and, critically, running with the very high reliability required for long-term high-speed autonomous operation – proved a nearly three-year process. ‘When you get into closed loop operation, you need an experimental setup that is reliable, robust, can continuously generate accurate data with minimum noise without breaking down,’ Abolhasani explains. ‘You have to automate every single task that you used to do manually.’ What little suitable hardware is commercially available was very expensive, so the team ended up building, validating and benchmarking five new modules for their automated system, from reactors to characterisation equipment.
But the results have been worth it, Abolhasani argues. In one of the team’s first studies, they set their artificial chemist the task of making a green-emitting quantum dot. ‘Exploring a space with more than 20 million possible experiments, without any prior knowledge, within 90 minutes it found a route to synthesise a semiconductor nanocrystal with the desired bandgap, optimised for its brightness and size distribution.’
The team also tested out their system with a potential industrial partner. The machine was set the task of discovering a synthetic route to a specific semiconductor nanocrystal the company makes, for which its chemists had spent 12 years working to develop a complex multi-step synthesis. ‘The chemistry that took them 12 years to develop, we discovered within one month,’ Abolhasani says. ‘To me this is the biggest value proposition. Just think about how much of the time of these highly trained scientists will become available to focus on solving new problems.’ Needless to say, the company asked Abolhasani to build them a self-driving lab, which they are now using it in their R&D facility.
Chempiling a chemputer
For Lee Cronin at the University of Glasgow, UK, there are far more fundamental reasons to embrace robot chemists than to facilitate next generation high-throughput searches of chemical space. Digitising and mechanising the conducting of chemical reactions offers the unprecedented possibility to make running and reporting of any chemistry experiment something unambiguous, precise – and repeatable.
‘Most of chemistry is about “Hey, I’ve done this thing and its useful” – but it’s not useful, because I can’t reproduce it,’ Cronin says. It’s often not that the original chemistry was poorly done, he adds, it’s just the inherent uncertainties that inevitably creep in when reactions are performed and reported by hand. ‘You shouldn’t have to spend six months reproducing a known reaction. The fact chemists are still doing that is embarrassing,’ he says.

The transition to automated robotic labs offers the potential to make a drastic change to the reproducibility problem – if the correct fundamentals are put in place, Cronin says. ‘There is a foundation I’m building,’ Cronin says. ‘About a decade ago, I invented χDL. It is the first ever programming language for chemistry.’ The language uniquely encodes everything a chemist does, defining the reactor and then the main unit operations of the experiment, including adding reagents, heating or stirring. ‘It’s like HTML for chemistry, and the objective is that publishers will adopt it,’ Cronin says. ‘The last Science paper I published, 50 reactions done by robot were encoded in χDL, and you can download the χDL from the Science website and they work 100% of the time.’
Without a standard programming language underpinning it all, there is no point building any automation, Cronin says, because it doesn’t address the central problem that reactions are not encoded in such a way that they are repeatable. ‘The χDL is hardware-agnostic, it “chempiles” down to any hardware,’ Cronin says.
To prove the point, Cronin and Hein just collaborated on a project they are calling molecular teleportation. Although the robotic hardware in Cronin and Hein’s labs is different, an χDL file generated by one can be executed by the other, Hein says. ‘I can call up his system and say “Go make this molecule for me”, and it will make it in Glasgow,’ says Hein, ‘and likewise he can beam me code and say “I want this molecule made in Vancouver” and boom it’s there. We’re beating UPS.’
‘It’s like Jason has an AMD processor and we have a Mac processor, but we can use the same code and it chempiles down to the right hardware,’ Cronin adds.
On the hardware side, for Cronin, the critical aspect is to keep costs as low as possible, to enable widespread adoption. Cronin now has over 50 different ‘chemputer’ robots running in his lab. ‘They all cost less than about £20,000 a go, and most of the cost is the third-party peripherals like rotary evaporators, stirrers. We even make our own pumps and valves. And we are slowly getting adoption.’
One key element of the progress his team has made is the diverse range of scientists he has been able to bring into his group, Cronin says. ‘Since 2010, Glasgow allowed me to hire PhD students from mathematics, computer science, engineering and physics, not just chemistry,’ he says. Students with different backgrounds work closely together on different projects. ‘Chemists need to let in more disciplines immediately,’ Cronin says.
Chemistry that fits
Lab automation offers fertile ground for chemists to interact with other disciplines, or even with non-scientists, says Martin Burke, a chemist at the University of Illinois, Urbana–Champaign, US. ‘We have barely scratched the surface of what we can do with molecules, and I think the key is to finally figure out how to empower everyone to find them,’ Burke says. Other fields that have brought non-specialists and even citizen scientists into the fold have seen real benefits, he says.

‘Computer science is a great example,’ Burke says ‘There is this program for learning coding, called Scratch Junior, which I learned about because one night at dinner, my kindergartner was laughing at me because I don’t know how to code. I’m thinking, “Wow, how do we get it to have kids laughing at their parents because they don’t know how to make molecules?” This is the dream.’ Other examples include using technology to empower citizen scientists to identify exoplanets or fold proteins.
Burke’s approach to robotic synthesis is to automate a select handful of reactions, which are used to snap molecular building blocks together, so that organic synthesis becomes more like making DNA, peptides or saccharides. ‘Everybody is trying to use AI for automated synthesis, but the automated synthesis is still the bottleneck if you are trying to automate every possible reaction,’ Burke argues. ‘We are saying, let’s just take two or three reactions, make them really good. Instead of developing AI for synthesis, we are trying to develop synthesis for AI.’
The core reaction Burke uses to link different small molecular building blocks together is a boronate N -methyliminodiacetic acid cross-coupling reaction. The method, which forms new sp2 carbon–carbon bonds, gives good access to polyheterocyclic materials. The approach is limited in terms of structure, but not of function, Burke argues.
‘Chemistry is a very structure-focused discipline,’ Burke says. ‘What I think would be very powerful is if we flip it and start to think of function first. We are trying to build a machine that can build any function.’
Looking to nature suggests this approach should be viable, Burke suggests. ‘For example, there are many different natural products that bind to microtubules and stabilise them to kill cancer cells, and those molecules couldn’t look more different.’
The right tool in the right place for the right reason
Burke originally developed his modular Lego-like approach to exploring functional space while looking for analogues of amphotericin as a possible treatment for cystic fibrosis. ‘This molecule was known to self-assemble and form ion channels in yeast and human cells, which we thought this might be enough to replace the missing CFTR ion channel in patients.’ The team developed their modular synthesis approach to discover analogues that retain the desired bioactivity but shed amphotericin’s infamous toxicity. A derivative they discovered is about to enter human clinical trials.
The team is now exploring whether using lab automation to chase function rather than structure is generally applicable – while also aiming to further develop the underpinning chemistry, by finding cross-coupling conditions that can generate stereospecific sp3 carbon–carbon bonds as efficiently as they can currently generate sp2 carbon–carbon bonds.
‘If we can deliver modular chemistry, that almost always works, to AI – well now, look out,’ Burke says. Because they are only trying to automate a reaction or two, and then just repeat them over and over, the robot part becomes easy, Burke says.
Across the diverse array of approaches chemists are now taking to combine AI and robotics in the lab, there is no ‘works best’ solution, says Hein. ‘Andy Cooper’s system is a really good way of taking well-built infrastructure and then building something around it that can flexibly work within that space,’ Hein says. ‘Lee Cronin’s focus, that we need a standardised system that anyone can use that covers 90% of all chemistry, is again a brilliant call to arms around that part. I think what you’ll see is different deployments – the right tool in the right place for the right reason.’
The community is competitive, but also highly collaborative. ‘We do have differences of opinion, because we come from different perspectives, but as a community we all acknowledge there is so much work to do that all of us is better than one of us,’ says Hein. The collaborative spirit reflects the fact that each came into the field as chemists trying to solve specific research problems, he adds. ‘We all backed into it, and hit shoulders,’ he says. ‘We are all learning this as a second trade, and so it’s like the hobby group teaching each other things that we have learned.’
James Mitchell Crow is a science writer based in Melbourne, Australia
How will AI and automation change chemistry?

It’s going to change our lives. But it’s not clear in what ways
- 1
 Currently
reading
Currently
reading
The robots revolutionising chemistry
- 3
- 4
- 5
- 6
- 7
- 8
- 9




























No comments yet