How do healthcare products get from academic or start-up labs to the clinic? With a little help from places like the Centre for Process Innovation…
The pioneering first moments in scientific discovery can enlighten, excite and promote wonder about the future of healthcare. Yet something that is not so often celebrated is the innovation required to turn these first stage realisations into the tangible new products that improve quality of life, cure the incurable and save lives.
From technology transfer offices in universities to crowdfunding and apps that connect businesses to research groups, multiple systems are attempting to marry the initial stages of discovery to business propositions. However, simplifying innovation to just being the direct result of serendipity would be doing the process an injustice. Each step along the innovation journey is unique, complex and requires addressing many different factors if commercialisation is to be achieved.
No matter how good an idea is, whether it claims to rewind ageing or eradicate cancer, it will need much long-term assistance and advice to successfully deliver on its innovation journey from research to commercialisation. Research breakthroughs that can kill cancers or improve dementia symptoms in the lab are all well and good, but without the innovation process to translate this research into viable products to treat such diseases, the benefits of scientific discovery on health would never be truly actualised.
Unfortunately, this integral innovation process is often difficult for many start-ups and SMEs with revolutionary ideas, as they have to secure the necessary funding and suitably manage risk for investors and other stakeholders. Because of this, the daunting process is often a journey that is far too complex and risky to embark on when commercialisation isn’t guaranteed. However, with the birth of dedicated innovation centres, like the Centre for Process Innovation (CPI) in the North East of England, these woes can be dramatically reduced for innovative companies with great ideas, increasing the chances of the next healthcare breakthrough reaching the market.
CPI works to reduce risk in the innovation process by supplying state-of-the-art equipment and expertise in process design, improvement and scale-up, to ensure products and processes that improve many people’s lives can be realised. In the healthcare space in particular, multi-industry innovation centres such as CPI are integral to delivering advanced health therapies that help us get treated more efficiently, recover quicker and live longer, healthier lives.
Listed below are just five of the most promising advances in technology for health that have been made possible thanks to CPI’s innovation support. Whether this is expanding an SME’s workforce to industrialise a technology, bringing companies together to deliver cross-sector solutions, or providing equipment to assist with scale-up, the innovations below show how CPI’s support is integral to navigating the perils of commercialisation to bring companies and their life-saving ideas to market, quicker.
Improving radiotherapy
In theory, most cancer types could be cured by radiotherapy – and it is used in the treatment of over 50% of cancer patients. But, inconveniently, the dose is limited due to its toxic effect on nearby healthy tissues that may result in severe organ damage and life-long discomfort.
Another disadvantage of radiotherapy is that it cannot be used to effectively treat certain aggressive cancers due to lack of oxygen in the tumour – a key ingredient needed for the x-ray or proton beams to induce cancer-killing free radicals.
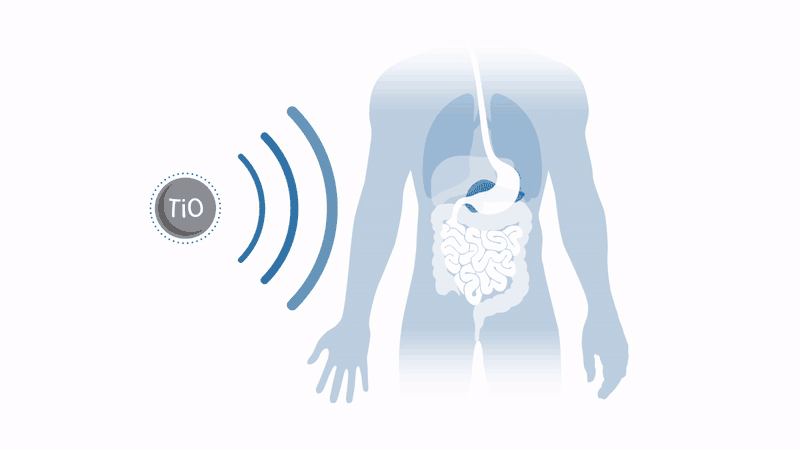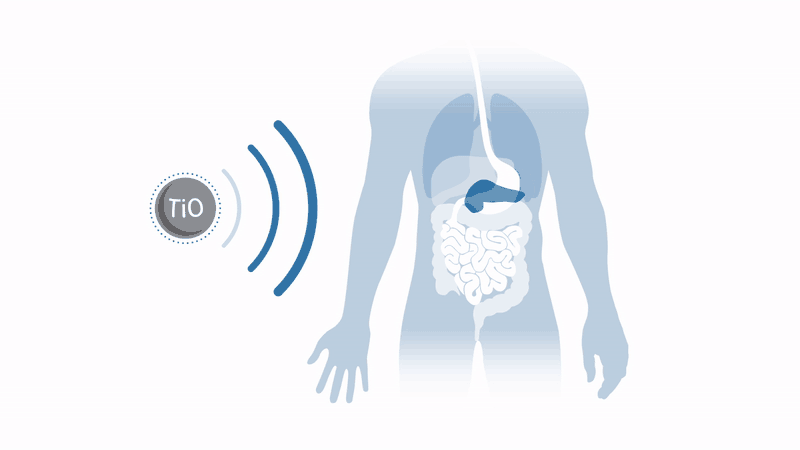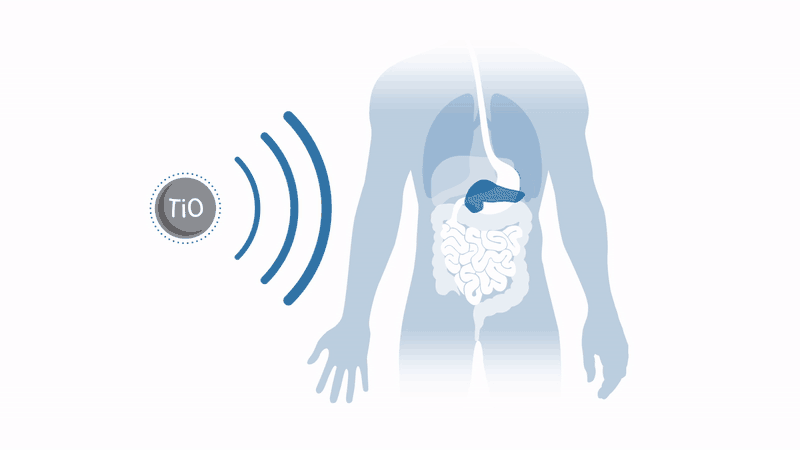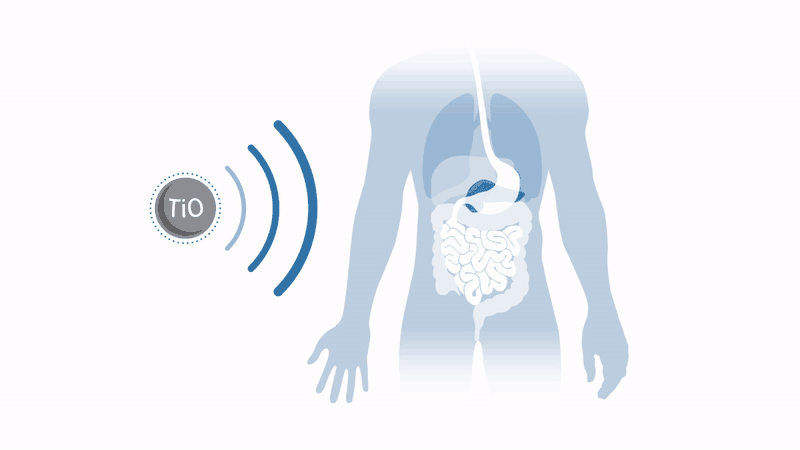
Working to overcome these two issues with the therapy, a company spun out of the University of Oxford in the UK, Xerion Healthcare, aims to commercialise a radiotherapy enhancer which dramatically amplifies radiotherapy response in a broad range of tumours. Xerion’s titanium dioxide nanoparticles, doped with specific rare earth metals, absorb energy from x-rays to efficiently generate cancer-killing free radicals by splitting water – without the need for oxygen.
This means that harsh tumours in regions lacking oxygen, such as the pancreas, can be treated, and that tumours in toxicity-sensitive regions, such as the head and neck, can receive amplified radiotherapy without damaging healthy tissue.
These nanoparticles have already demonstrated a dramatic increase in the effectiveness of radiotherapy treatment in mouse models in vivo and in vitro. Throat cancer tumours injected with nanoparticles have seen regrowth suppressed by a factor of four after conventional radiotherapy. Even better, Xerion’s nanoparticles can be used in conjunction with standard radiotherapy equipment and do not require major changes to existing clinical practice.
With clinical trials set to begin in 2020, it may not be long before radiotherapy becomes the go-to reliable cure for cancer, ahead of invasive surgery, avoiding the life-limiting side effects that have become so normalised for today’s cancer patients.
Devices to remove human error
The ‘never event’ list, used by the UK’s National Health Service, is comprised of very serious patient incidents that should never happen if relevant preventative measures have been put in place. But sadly, these ‘never events’ sometimes do happen.
The latest advances in automation offer an opportunity to eliminate human error in ways similar to how we are seeing driverless cars and robot-run factories become reality. Whatever your stance on this fast-approaching futuristic world, there is no doubt that harnessing technology to remove human error in healthcare applications could save time and money, as well as lives.
One device currently being developed may well eradicate one of these ‘never events’ for good – feed or medication administered via a misplaced nasogastric (NG) tube. These soft, thin tubes are commonly inserted through the nose of patients into the stomach. Mistakenly implanting this tube into the lungs can have horrific consequences for patients, such as feed being administered to the incorrect location, and is known to have caused 13 serious injuries and deaths in the UK between April and October 2017. At present, every nasogastric tube inserted needs to be checked that it is in the correct place through an x-ray, before every feed. This is an incredibly lengthy and expensive process considering the 800,000 NG tubes that are sold each year, and that many patients often have them fitted for many days at a time.
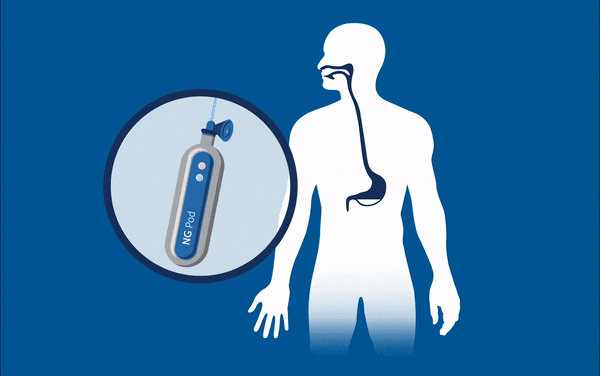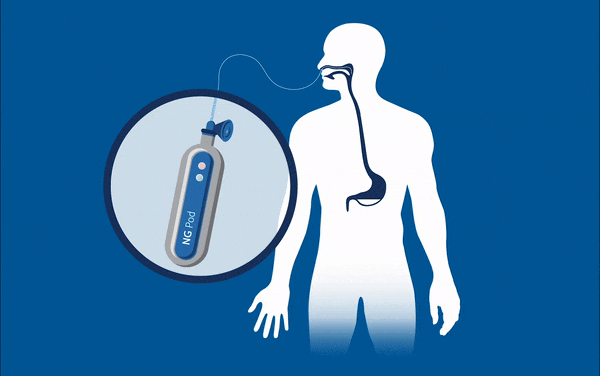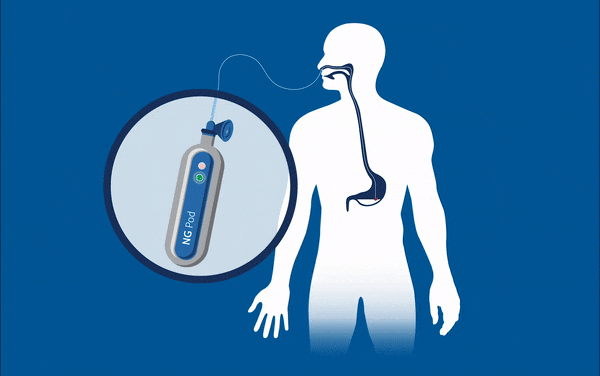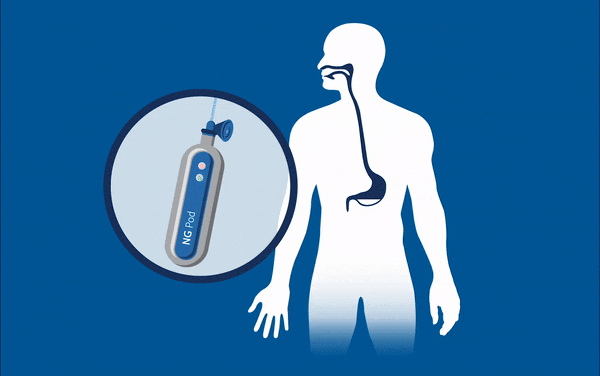
Removing the need for interpretation, the NGPod bedside diagnostic device gives clinicians a yes or no result as to whether a NG tube is correctly placed in the stomach. It works, quite simply, by a small disposable fibre optic sensor device that detects pH, attached to a small hand-held light source. The tip of the sensor houses a chemical indicator which changes colour at a pre-determined pH level. If the NG tube is correctly placed in the stomach, where the environment is of a considerably lower pH to other areas, the indicator will change to a green colour, which is then interpreted as a ‘yes’ reading on the device.
The device gives clinicians the sound judgement about its correct placement and, by bringing this process to the bedside, could dramatically speed up treatment and reduce costs for healthcare providers, and may eliminate one ‘never event’ for good.
Nanoparticles advancing imaging
Getting a diagnosis used to be very different. If you were unfortunate enough to be ill in ancient Sumeria, your physician was more likely to sacrifice a goat and ‘read’ its liver than check your blood pressure. Even as recently as 1700, European doctors were diagnosing gall bladder stones by checking for Scorpius in the night sky. But fortunately for all of us, medicine has since moved on. With the inventions and advancements of diagnostic tools such as the microscope and the stethoscope, interpreting the clues of illnesses started to become an actual science.
But some things still stood in the way of diagnoses. Like skin – if only there were some way to see inside the human body and really know what was going on. Thanks to Wilhelm Röntgen and the subsequent invention of x-ray radiography, it wasn’t long before we could do just that. The field of bioimaging was born.
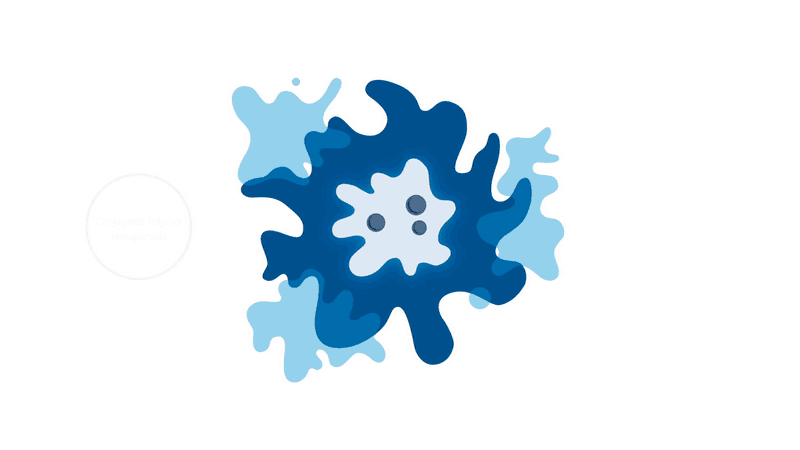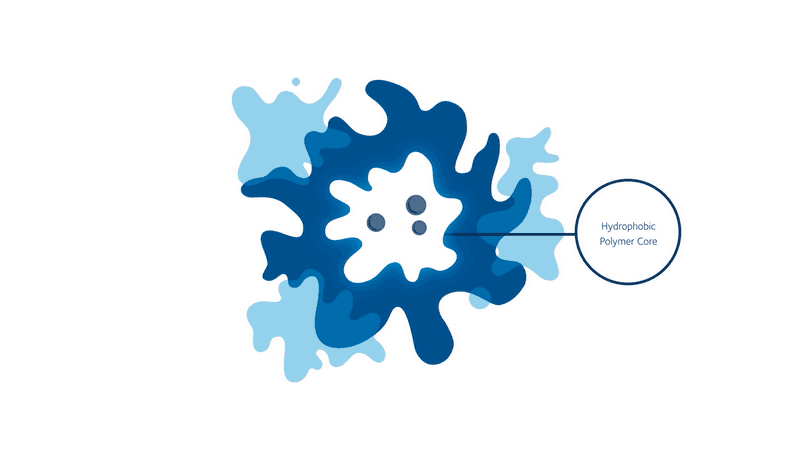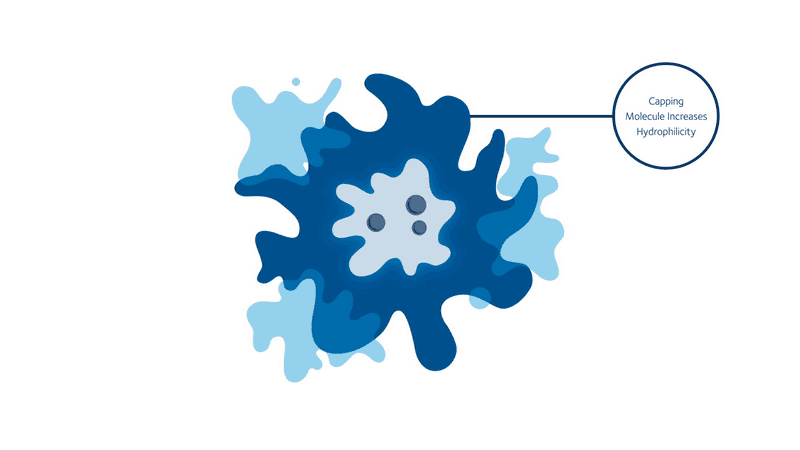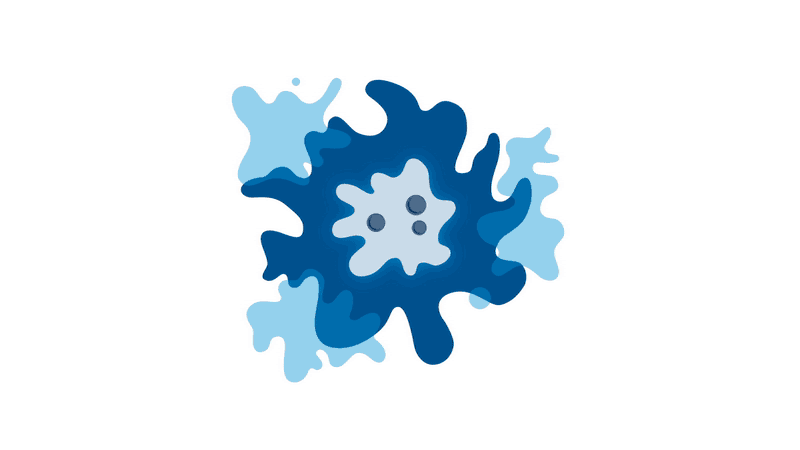
But progress never stops, and we may soon have a revolutionary new way to ’see’ into the body. Initially discovered at Kings College London in the UK, scientists from Stream Bio have further developed a unique conjugated polymer nanoparticle (CPN) that could one day replace the existing fluorescence markers that are used in routines such as cell imaging and cancer tracking.
These CPNs can be used in research to manipulate cell samples, track and target diseases, and eventually move around the bloodstream like tiny doctors, lighting up tumours for the surgeon to accurately cut out, or delivering a particular drug straight to where it matters. And unlike other conventional bioimaging fluorophores, these CPNs can retain their luminosity for nearly two years and come with the added bonus of being non-toxic (unlike cadmium-based quantum dots), meaning they can be used in human safety trials.
When it comes to transforming medical practices, researchers are always told to think big. But seeing as CPNs have the potential to improve patient waiting times and the diagnoses and treatment of a range of diseases, sometimes it never hurts to think small, too.
Accelerating DNA therapies
At the World Economic Forum in Davos, Switzerland, in January 2017, it was announced that the Bill and Melinda Gates Foundation is contributing to a $460 million (£330 million) fund to prepare for future epidemics. The coalition partners include the German, Japanese and Norwegian governments as well as the Wellcome Trust. Set up in response to the Ebola outbreak, which claimed over 11,000 lives in West Africa over only two years, the global partnership, known as CEPI, aims to prevent similar future epidemics by producing new vaccines.

When a new epidemic occurs, CEPI is aiming to produce a vaccine for it, and to have scaled it to 100,000 doses in only eight weeks. But this is currently nearly impossible with conventional technologies. One possibility identified by Gates was to use nucleic acids (DNA or RNA) to deliver the genetic code needed to vaccinate against the emerging outbreak. DNA is increasingly being used as a next-generation medicine across the biopharmaceutical industry (in gene therapy, genome editing and vaccines, for example). However, there is a common issue across industries that are applying DNA.
At present, DNA is amplified using a decades-old process: first inserting it into a bacterial cell, growing it in a fermenter and finally extracting it. DNA is the future – we’re using it for revolutionary next-generation healthcare products, such as personalised cancer vaccines to immunise individuals against their own cancer. Yet due to this cumbersome and slow process, this approach is limiting the progress of these innovative industries.
However, it may not be too long before the production of DNA-based products is transformed, as companies are beginning to commercialise innovative and advanced DNA technologies. One such company is Touchlight Genetics, which has developed a unique DNA vector known as doggybone DNA (dbDNA). This is a minimal, closed-linear vector produced using revolutionary enzymatic technology, which is immensely faster and can be scaled to meet growing demand.
Commercialisation of Touchlight’s enzymatic amplification platform is imminent, so it will not be long before every major limitation of the incumbent DNA fermentation technology is addressed. What’s more, Touchlight is collaborating with pharmaceutical companies in order to enable a wide variety of therapeutic modalities to benefit from this healthcare technology. Maybe Bill Gates’ standards for epidemic preparedness really will be realised sooner than we think.
Electronics to monitor medicine delivery
In the UK alone, it is estimated that £300 million is lost each year due to unused medicines; in the US, this number is as large as $2 billion. In a time of strict medical budgets and heightening concern over plastic waste, the problem of unused medicine is starving healthcare providers of valuable resources and must be solved sooner rather than later.

Fighting to put an end to this predicament is the concept of smart packaging – a way of integrating electronics into the packaging of medicines such that location, whether a blister packet has been broken, and even the temperature of medicines can be monitored. If properly invested in, the impact of smart packaging on the whole medical supply chain could be dramatic. From improving patient life expectancy by reminding patients to take their medicines, to digitisation of hospital management and automating the supply chain by delivering drugs exactly when and where they’re needed.
This sounds incredibly complex, but with the recent dramatic developments in printable electronics, elements of smart packaging could soon be made a reality, simply by updating the barcode system. Working to do this is internet of things company, M-Connected, that has recently branched out to revolutionise the world of health using its technology. M-Connected’s hyperlabels have embedded GPS technology that can detect exactly where a drug is in the supply chain – it has essentially taken part of a smartphone and inserted it into a thin, flexible, disposable label. The development of this smart packaging technology proves how integrating and connecting companies across sectors, from electronics to freight and logistics, to chip manufacturers and pharmaceuticals, can address historical healthcare dilemmas and deliver future-proof, digital solutions.















No comments yet