We take it for granted that the personal care products we buy are safe, and free from microbial contamination. However much care is used while they are being made, if a product is non-sterile, then bacteria or fungal organisms are likely to be present. We hear from the experts at Sartorius about how manufacturers can use membrane filtration to test for unwanted microorganisms.
It is essential for manufacturers to check the levels of microbes a product contains, and that none of them are particularly dangerous. This can be achieved using two test protocols laid out in the US Pharmacopeia, USP <61> and USP <62>. USP <61>, assesses the overall quantity of bacteria, yeasts and moulds that are present in a product sample. It is designed to be used on non-sterile pharmaceuticals, such as cough syrups and nasal sprays, and on raw materials used in manufacturing sterile pharmaceuticals. But also covers personal care products and nutraceuticals – anything that is designed to be ingested, inhaled, or applied to the skin.
The second test, USP <62>, looks to identify specific bacteria, yeasts or moulds in a sample. The way a product is used will determine which of these microorganisms might be problematic. Both tests include instructions about how samples should be prepared, and how the tests should be run.
Requirements in the EU, Asia and most other regions are largely similar, the outlier being China, where demands are a little different. However, they are all fairly well aligned, with the different pharmacopoeias having made efforts to harmonise their requirements. The differences are largely in the way the requirements are expressed. Therefore, the USP is a good guide to what needs to be tested.
products you might think cannot be filtered actually can
Myriam Gueye
In order to carry out these tests, the bacteria, yeast or mould have to be isolated from the product, getting rid of anything that could inhibit their visibility, and concentrating them to make them easier to spot. ‘The protocols specify that if it is possible to isolate antimicrobial agents using filtration, this should be done before the testing is done, and membrane filtration is preferred,’ says Tricia Vail, regional business manager at Sartorius.
‘Filtration, particularly for cosmetic and personal care products, provides a simple way of removing any preservatives that might be present,’ says Vail’s colleague Myriam Gueye, segment manager at Sartorius. ‘These are specifically designed to inhibit microbial growth within the product, and if they remain in the test sample, clearly an accurate result will be impossible.’ By using membrane filtration, and then rinsing the filter, any preservatives should pass through, leaving the microorganisms ready to grow without any inhibition.
Membrane filtration
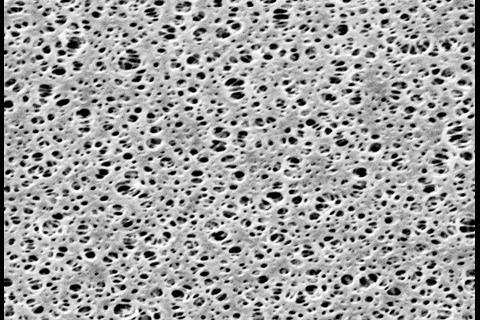
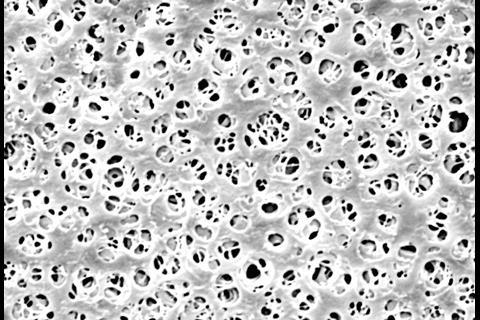
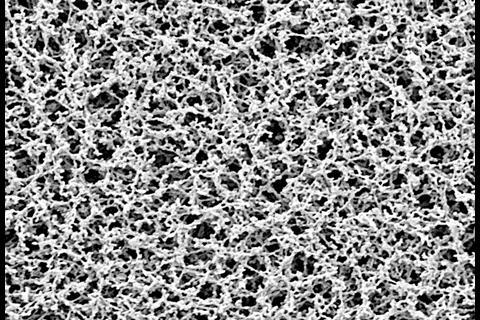
The process of membrane filtration is simple: a specific volume of liquid is pulled through a filter, which retains anything that is larger than its pores, normally 0.45µm. This is sufficiently small to catch any bacteria, yeast or mould within the product. The filter is then transferred to some form of culture medium, usually an agar plate,. This is then incubated for an appropriate length of time before the microorganisms are enumerated and/or identified. In some cases, a soluble filter can be employed, facilitating PCR testing.
Membrane filters with a pore size of 0.2µm are also sometimes used for personal care products, Vail says, but 0.45µm is more common. ‘You might think it’s an advantage to get better recovery with the smaller pores in the 0.2µm filter, but they tend to grow less well as their access to nutrients in the medium can be restricted,’ she says. ‘The growth rate is not the same, and it can be easier to miss them. Most of the regulations specify 0.45µm filters, apart from a very few microorganisms where 0.2µm may be necessary.’
the fewer bacteria the product contains, the longer it can be stored safely
Myriam Gueye
The membrane filters themselves can be made from many different materials. Cellulose nitrate and cellulose acetate are commonly used, as are polyvinyl and polyether membranes. It is important is to select a filter that is compatible with the chemistry of the product, so that the bulk of the product passes through unhindered by interactions with the material, and the filter remains undamaged. Components such as antibiotics might bind tightly to cellulose-based membrane filters, for example, so these should be avoided for products that include them to prevent false negative results. ‘We need to make sure the bacteria, yeast and moulds stay intact, and do not change the configuration of the membrane,’ Vail says.
Of course, a few products are not suitable for membrane filtration, perhaps because they are too thick to pass through easily, or are too pulpy or lumpy. If thickness is the issue, then it may be possible to dilute the sample sufficiently to make the filtration possible. But, Vail says, even if the product looks clear and is not thick, filtration may fail. ‘It might be loaded with proteins, and that would not be filterable as the proteins do not pass easily through the pores,’ she says. ‘But other products you might think cannot be filtered actually can. Petroleum jelly is a good example – all you need to do is heat it up gently, and it becomes a liquid that will filter.’
What’s in it?
While the tolerance for presence of microorganisms in a non-sterile product is clearly not as strict as it is for something sterile that is to be injected, clearly the levels should still be low, and nothing particularly dangerous should be present. ‘You don’t expect a shampoo to be sterile, but equally you don’t expect it to contain candida,’ Gueye says.
a system such as Microsart from Sartorius is designed to make the membrane filtration process simpler and safer
Vail agrees. ‘Tap water contains bacteria, but we can handle a certain amount of them, depending on what they are, as they will be destroyed by the acid in the stomach,’ she says. ‘Our skins can also cope with bacteria, but you don’t want your face cream to contain MRSA.’
The same tests are required for any non-sterile raw materials that are used in the manufacture of sterile products, such as water, salts or gelatin powder – this, in particular, is often heavily contaminated with bacteria. ‘It is important to know what is in these products before they start the manufacturing process,’ Vail says. ‘If bacteria, yeasts or moulds are present, they may need to change their sterilisation method, or add another step such as a membrane filtration. They need to know what the bacterial or fungal load is at the outset so they can ensure patient safety.’
The level of microbial contamination will also affect the final product’s shelf-life, Gueye says. ‘The fewer bacteria the product contains, the longer it can be stored safely,’ she says. This is also an important consideration for international shipping. ‘If you have been following strict regulations, you can be more confident that they will be approved in the country where they are being shipped.’ It also reduces the likelihood of recalls, with all the additional costs and reputational harm that these can cause. While preservative levels can be adjusted to keep the levels in check, she says, with the customer demand for products with no preservatives, ensuring the products are not contaminated in the first place is increasingly important.
Filtration in practice
A system such as Microsart from Sartorius is designed to make the membrane filtration process simpler and safer, using Microsart@filter filtration units. The Microsart manifold itself is has an ergonomic low profile, and is made from 316L stainless steel to facilitate decontamination. Different types of filter funnels can be used by simply changing the adapters on the manifold. It is even possible to use black filters rather than white ones, depending on the colour of the contamination, making it easier to see.
Disposable funnels are commonly used to minimise the chances of cross-contamination of samples. Another potential source of cross-contamination comes from the forceps used to move the filters to the petri dish for incubation; the system’s Microsart@media plates containing media can be attached directly to the filter, thus avoiding manual handling. Forceps are typically dipped in alcohol after use to remove any contamination, but if this is not allowed to dry off fully, it can spread across the membrane, killing the organisms and leading to a false negative result and, potentially, a product recall later on. ‘I’d take a thousand false positive investigations ahead of one false negative,’ Vail says.
Want to discover more solutions for beverages? Please visit the Sartorius website.
Continuous microbial air monitoring in clean rooms
Get a free study from Sartorius on the the use of gelatin membranes for continuous air monitoring in industrial pharmaceutical production environments throughout an eight hour work shift.
















No comments yet