Separating enantiomers is crucial when it comes to making drugs as left-handed and right-handed mirror image molecules can have very different effects in the body. Most chemosynthetic processes naturally produce racemic mixtures, however, and separating different enantioners can be tricky. But now researchers in China and Korea have created a self-assembled membrane that selectively absorbs one enantiomer from solution, before releasing it on the addition of a salt.
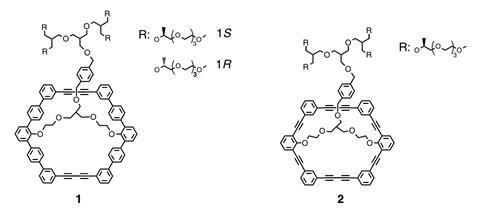
Myongsoo Lee of Jilin University in Changchun, China and colleagues were working with a molecule with a buckled aromatic macrocycle containing 11 aromatic rings and a protruding oligoether dendron with two enantiomers. The researchers found that when they dissolved these molecules in methanol they formed dimers, with the inward-facing macrocycles fixed at relative orientations of 60°. The orientation of the macrocycles depended on which enantiomer of the dendron was attached. Intermolecular forces between the dimers caused them to self-assemble into extended two-layer sheets, with hexagonal symmetry within each layer and a 60° twist angle between the layers – effectively forming a grid of homochiral nanocavities. The outwardly-protruding dendrons – which were strongly attracted to the methanol solvent – prevent stacking.
When the researchers added a racemic mixture of a derivative of the amino acid tryptophan to a solution containing their sheets, they found that one nanosheet enantiomer captured L-tryptophan with 96% efficiency, while showing absolutely no affinity for D-tryptophan. The other nanosheet enantiomer captured D-tryptophan with the same efficiency and selectivity. The sheets showed no apparent affinity for derivatives of several other amino acids, which could make them useful for sorting enantiomers and molecules in one step.
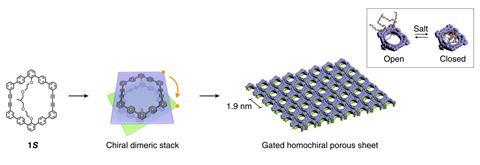
Lee says the 2D nature of the membranes provides a significant advantage, as 3D chiral pores designed to capture multiple molecules at once can become distorted by molecules already in the pores. ‘In our 2D system, one guest binds to one pore to demonstrate exactly the same interaction between guests and pore walls,’ he says. ‘This approach can contribute to highly-efficient enantiomer sieving membranes which selectively adsorb enantiomers with only one discrimination event.’ After the sheets had captured the desired isomer, the researchers added ammonium acetate to the solution. This drew solvent molecules away from the dendrons. The dendrons consequently collapsed into the pores, releasing the trapped tryptophan derivative.
William Dichtel of Northwestern University in Illinois, US is impressed. ‘It’s a brilliant example of amplifying a very small molecular chirality element up to a much larger system,’ he says. ‘In terms of thinking about it as a true future technology for enantioselective separations – I wouldn’t go that far yet. If you could get transport through these membranes and run them continuously that would be more promising technology-wise. But that certainly should not take away from how beautiful this work is.’
References
B Sun et al, Nat. Mater., 2018, DOI: 10.1038/s41563-018-0107-4













No comments yet