86-year old Hammett equation gets a machine learning update
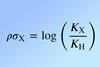
Algorithm opens the door to improved understanding of aromatic substituent effects
The Hammett equation, a chemical theory that is over 80 years old, is being expanded upon and improved with the help of machine learning. The equation, which can help to explain the electron-donating or withdrawing nature of aromatic substituents via calculation of Hammett constants, has been analysed computationally by a team of Brazilian researchers who want to make it even more precise, and unlock unknown values for practical experiments.
‘There are some experimental Hammett’s constants which, although widely used in many applications, were not measured or have inconsistent values,’ says Itamar Borges Jr from the Institute of Military Engineering in Brazil who worked on the study alongside Julio Cesar Duarte and Gabriel Monteiro-de-Castro. He adds that the work ‘employ[s] machine learning algorithms and available experimental values to produce a consistent set of the different types of Hammett’s constants’.
