Researchers break record for benzene ring with the largest twist angle
A research group from Japan has made a substituted naphthalene that contains the most twisted benzene rings ever synthesised.
According to Erich Hückel’s 1931 rule, a molecule can only be aromatic if it has a certain number of p electrons that are delocalised around a planar ring. However, researchers have been pushing this rule to the limit by synthesising sterically hindered systems that twist aromatic rings out of planarity.
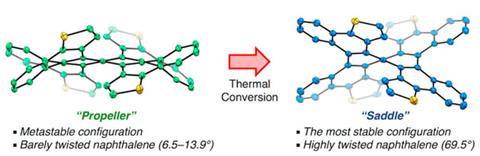
Having previously discovered how to easily synthesise non-planar aromatics, Kenichiro Itami and his Nagoya University team were exploring helical molecules by fusing various aromatic rings to a central naphthalene core. When one of their reactions didn’t go as planned, they found that they had made a new propeller-shaped molecule that interconverted into a twisted saddle-shaped stereoisomer when heated. With an almost 36° twist angle, this isomer’s central benzene rings have a 6° higher twist angle than the previous record holder.
By studying distorted aromatic systems the team hopes to ultimately develop new functional materials.
References
T Fujikawa, Y Segawa and K Itami, J. Am. Chem. Soc., 2016, 138, 3587 (DOI: 10.1021/jacs.6b01303)

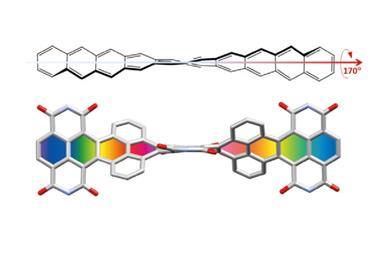
![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)







No comments yet