Amorphous clusters of amino acids and peptides formed in solution with sizes ranging from the molecular to micrometre scale influence crystal nucleation, according to research by a team in the UK.1 This new insight could allow scientists to better understand and control crystal growth of a range of compounds.
In classical nucleation theory, an energy barrier must be overcome to go from one phase to another. For example, to form a crystal from a solution, individual molecules attach to a crystalline nucleus when it reaches a critical size. ‘In recent years, theoretical and experimental studies had indicated that the nucleation mechanism is more complicated than envisioned in classical nucleation theory and involves the formation of amorphous clusters,’ explains Allan Myerson, an expert in crystal nucleation from the Massachusetts Institute of Technology, US, who wasn’t involved in the research. However, the nature of these clusters is largely unexplored.2
To investigate this notion, Klaas Wynne at the University of Glasgow and Rebecca Beveridge at the University of Strathclyde teamed up to explore the size distribution of aggregates formed in supersaturated solutions of amino acids and peptides. First they observed that amorphous nanoclusters form over several days, using dynamic light scattering. The data could be analysed using a stretched exponential function, which suggests a range of structures of different sizes were present. Next, Beveridge’s team used mass spectrometry to image small nanoclusters that aren’t visible using dynamic light scattering techniques. ‘It was really nice to be able to add an extra arm to the analysis and to look at all of the smaller things, and it was really complementary to the larger things Klaas was able to look at,’ says Beveridge.
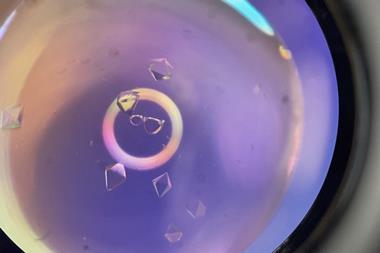
They discovered that the aggregates, which vary in size from dimers up to the micrometre scale, play a crucial role in crystal nucleation. ‘Instead of overcoming a big barrier associated with this big interfacial tension of making a small crystal, you circumvent it by making an amorphous structure which has a very low surface tension,’ Wynne explains. The aggregates provide a site for nucleation, which lowers the energy barrier to crystal formation. Indeed, the team observed both spontaneous and laser-induced nucleation of crystals from the aggregates under a microscope. ‘[This study] demonstrates that the aggregates have a size distribution which influences the transition to crystalline nuclei,’ confirms Myerson.
The team next plan to use alternative spectroscopies to visualise clusters on a length scale between those seen by dynamic light scattering and mass spectrometry. ‘I personally would like to use this [new knowledge] to start controlling things,’ says Wynne, who says the findings could be expanded to pharmaceutical, inorganic and even protein nucleation. ‘That molecular scale processes on a timescale of terahertz or picoseconds have an effect on something that happens on the scale of hours or weeks is impressive.’
References
1 Liao et al, Chem. Sci., 2024, DOI: 10.1039/d4sc00452c
2 Svärd et al, CrystEngComm, 2022, 24, 5182 (DOI: 10.1039/d2ce00718e)




















No comments yet