Anti-electrostatic halogen bonding shown in solution for the first time
UV-vis spectroscopy and computational analysis explore nature of counterintuitive anionic interaction
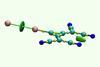
An international team of scientists has identified a series of halogen-bonded complexes formed by two anions that are stable in solution. Spontaneous formation of such counterintuitive interactions shows that halogen bonding is strong enough to overcome electrostatic repulsion between two anions.
