Researchers in China and Australia have developed an adjuvant system that activates both innate and adaptive immunity by inducing programmed cell death in dendritic cells. The system uses sodium-stabilised dendritic aluminosilicate nanoparticles, which can carry antigens and have basic sites that trigger pH-responsive H+–Na+ exchange. Compared to commercial and clinical adjuvants in an in vivo colon cancer model, the nanoparticles triggered a stronger immune response.
Adjuvants are vaccine components that amplify the body’s immune response. The most commonly used adjuvant is alum, an aluminium salt. ‘Alum induces strong humoral immunity, but weak cellular immunity, therefore it is not suitable for vaccination against some infectious diseases and cancers,’ explains Chengzhong Yu from the University of Queensland.
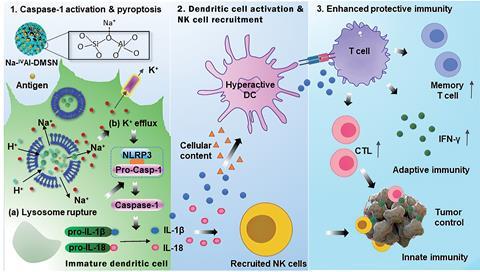
Now, Yu’s team has developed an adjuvant system that builds on research highlighting the importance of both innate and adaptive immune responses for vaccines. It ‘mimics the process by which natural viral infections occur, where pyroptosis [programmed cell death triggered by caspase 1] is induced in antigen presenting cells, eliciting protective immunity,’ says Yu.
Yu’s adjuvant system can also carry an antigen. Previous work from his group using mesoporous aluminosilicate nanoparticles as adjuvants allowed high antigen loading, but the nanoparticles did not trigger the intracellular ion perturbation that leads to pyroptosis. The team’s new system includes modulators to hyperactivate dendritic cells. It has basic sites, which allow H+–Na+ exchange. This ion exchange process ruptures lysosomes, and causes potassium efflux that ultimately leads to caspase 1-dependent pyroptosis of dendritic cells. Bystander dendritic cells then mature, which recruits natural killer cells and T cells, enhancing both adaptive cellular immunity and innate immunity.
The aluminosilicate nanoparticles were synthesised using a simple post-modification approach. First the team prepared and calcined mesoporous silica nanospheres in a one-pot process. Combining the nanospheres with aqueous sodium aluminate then produced uniform and non-aggregated nanoparticles.
Tests using ovalbumin, a model antigen, allowed the team to study the system’s loading capacity, release and delivery performance. They assessed its ability to induce cellular immunity against tumours in an in vivo prophylactic colon tumour model. Control tests using the commercial adjuvant Alhydrogel showed that Yu’s team’s aluminosilicate adjuvant more effectively decreased tumour growth. The team also observed encouraging results when investigating the humoral immune response that suggest their system would benefit vaccinations striving for cellular immunity and antibody production.
‘Proper choice of adjuvant is key to the efficacy of a vaccine,’ comments Quan Li, an expert in nanostructured materials with medical applications from the Chinese University of Hong Kong. ‘This is an exceptional piece of work that not only shows a novel material design for nanoadjuvants (at the same time, an antigen carrier), but also presents a clear pathway for the enhanced adjuvanticity.’











No comments yet