Atom-swapping reaction sequence turns cyclic diarylmethanes into diarylethers
Strategy that stitches three different reactions together could be useful for making pharmaceutical intermediates
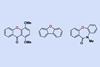
Chemists in the US have developed a strategy for creating cyclic diarylethers by replacing a carbon atom with an oxygen atom in the core framework of cyclic diarylmethanes and diarylketones. The approach uses a phosphine ligand with a nickel catalyst and could be useful for making pharmaceuticals containing a dibenzoazepine motif.