Bizarre bimetallic compounds break C–F bonds
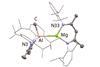
First ever molecular Mg–Al bond constructed in study that reveals main group metal–metal bonds can readily react with aromatic fluorine
Bimetallic main group complexes can activate aromatic fluorine, a study by researchers in the UK has found.1 The reaction has a mechanism very similar to a typical nucleophilic aromatic substitution with a Grignard reagent and provides a simple route to a number of very rare molecular species – including a new compound with the first ever molecular Mg–Al bond.