Bottleable but reactive carbocations are powerful hydride abstractors
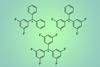
Fluorinated trityl cations outperform trityl for hydride abstraction reactions
Researchers in the US have isolated partially fluorinated trityl cations and demonstrated that they can act as powerful hydride abstraction agents for organometallic reactions.