Covid-19 has highlighted the challenges of storing vaccines at their proper temperature. Angeli Mehta explores how scientists are trying to protect vaccines from heat degradation

As the world celebrates the arrival of vaccines to protect against Covid-19, the logistics of distributing them is under the spotlight as never before. It’s a delicate balancing act – both heat and excessive cold damage vaccines. Keeping vaccines at just the right temperature to ensure they’re effective is an expensive business, with the cold chain accounting for up to 80% of the costs of delivery.
‘The agencies responsible for taking vaccines to the developing world spend more than $400 million [£290 million] a year just on refrigerators – just keeping the cold chain intact. That in itself would buy a lot of vaccines,’ says Bruce Roser, a biomedical researcher who works on vaccine stabilisation at the company he founded, Stablepharma, in the UK.
There have been innovations since the WHO estimated, back in 2005, that up to 50% of vaccines get wasted: refrigerators that can stay cold for days at a time in spite of power cuts and the successful roll-out of a vaccine for Ebola in west Africa that required storage at -60°C. The trial team used double walled drums filled with phase-change proprietary denatured alcohols that crystalised at -85.5°C. Even when used regularly at temperatures as high as 42°C, the containers called Arkteks could maintain temperatures of around -70°C.
That’s also the temperature required for storage of the world’s first mRNA vaccine – and the first to be approved for use against Covid-19. The French government suggested up to 30% of the vaccine developed by BioNTech and Pfizer could potentially be wasted, given the challenges of distributing it. However, a second mRNA vaccine from Moderna will survive in warmer storage conditions between -25 and -15°C, whilst another developer, Curevac, says its mRNA vaccine – currently in trials – will be stable in a fridge for three months and can cope with 24 hours at room temperature.

Messenger RNA instructs cells to make the spike protein that produces the immune response. To be safely transported into the cells, the vaccine is encased in lipid nanoparticle droplets. Curevac spokesperson Thorsten Schüller tells Chemistry World that its vaccine candidate differs most from other mRNA approaches because it uses natural, non-chemically modified mRNA, and that this defines its general architecture. ‘For the mRNA to remain stable within the lipid nanoparticle it needs to be protected from hydrolysis. We think we might have achieved this by having the mRNA tightly packed within the lipid nanoparticle. Our theory is that the more compactly the mRNA is packaged, the less attack surface there is for hydrolysis.’
Both BioNTech (and its partner Pfizer) and Curevac use lipid nanoparticle technology developed by Canadian firm Acuitas Therapeutics. Its president and chief executive Thomas Madden doesn’t think there’s anything inherently different about these three vaccines but rather, in Pfizer and BioNTech’s case, the time available to carry out stability studies to determine how long and under what conditions the vaccine can be safely stored. These studies might normally take as long as two years. ‘We simply didn’t have time to do that, obviously, for the vaccines being developed by BioNTech and Pfizer and we didn’t want to delay making the vaccine available and so the most conservative approach was to store it at minus 70°C, because nothing is going to happen to it under those conditions,’ says Madden.
The companies are now undertaking stability studies for storage at normal fridge temperature (2–8°C) and at -20°C to generate data that will be submitted to the regulatory agencies. Pfizer is also working on a freeze-dried – lyopholised – formulation, which it anticipates will be stable under normal refrigeration. So longer term, Madden is ‘reasonably confident’ that ultra-cold storage won’t be necessary.
Russia’s Gamaleya Institute, which developed the Sputnik V adenovirus vaccine, has already developed a powder form for ‘hard to reach’ areas of Russia, but says production would be biased towards its frozen vaccine (which requires storage at -18°C) because producing a lyopholised form is more expensive and time consuming.
Not all vaccines can be freeze-dried because in excessive cold, damage by ice crystals causes them to fall apart. Swedish-based Ziccum has developed a means of air-drying vaccines, and designed a fill and finish plant to produce them that it claims can operate far more cheaply than a plant making freeze-dried formulations.
There are some circumstances in which existing vaccines can be kept outside the cold chain for a limited time, in a Controlled Temperature Chain. The results of a study in Benin, using the vaccine developed against meningitis A led to its getting approval to be kept at 40°C for up to four days, cutting logistics costs in half. Other vaccines have similarly proven to be able to cope with higher temperatures for short periods, but ‘sometimes companies are reluctant to apply again to the regulatory bodies, because they don’t have much to gain. It won’t translate into higher market shares,’ says Julien Potet, Médecins Sans Frontières’s access campaign policy advisor. ‘For vaccines used in large-scale campaigns, where millions of doses are transported over a short period of time, any [such] improvement in the cold chain would be very valuable.’
Thinking ahead
There’s no shortage of research effort aimed at ditching the cold chain entirely, thanks to inspiration from plants that cope with extreme drought to developments in biocatalysis.
Jason Hallett’s group at Imperial College London, UK, working on sustainable biofuel production wanted to get their enzyme working at higher temperatures, to speed up the process of breaking down cellulose. They found that by using ionic liquids – salts that are liquid at any temperature – they could stabilise the enzyme at very high temperatures. Hallett wondered if the same approach could be used for vaccines, so they could have a shelf life of months or years – even in the tropics.1
For vaccines based on proteins, the formulation the Imperial team has developed stops the protein from folding and unfolding as it gets heated up. It also prevents the proteins from clumping together – which they are prone to do at the concentrations required for their use in vaccines. ‘Basically it can breathe a little bit, but it can’t really unfold because there’s too much surface charge or put another way, there are too many ions around,’ explains Hallett. With RNA-based vaccines they stabilise the lipid delivery vehicle. ‘The stabilisation approach is very similar, but we do not need to do any surface engineering. We simply use the ionic liquids as formulation aids by changing the way they interact to prevent aggregation. We also gain some benefit by slowing hydrolysis of the RNA itself.’
Hallett’s group has reformulated a self-amplifying RNA vaccine being developed against Covid-19 by colleagues at Imperial. It’s stable at room temperature for at least 50 days – that’s how long they were unable to access the lab during last year’s first lock down. The vial was simply left in a drawer. ‘The biggest challenge that we had was actually not a chemical one, because it was relatively straightforward to adapt our enzyme formulations to vaccines – but I wasn’t sitting there thinking we were going to inject ionic liquids into people,’ says Hallett. To come up with a non-toxic formulation the team has turned to molecules found in foods and used these to create a new salt. However, they’re not ready to inject these into a person quite yet.
Bruce Roser’s journey began with the resurrection plant – so named because it can survive without water, almost completely desiccated. This is thanks to its capacity to make a sugar – trehalose – that protects the plant as it dries out. When water is available the plant unfolds and begins to grow again. ‘Trehalose turns out to be the ideal stabilising agent for long-term high temperature storage of pretty well anything,’ says Roser. ‘Most of the things that the pharmaceutical industry has used to stabilise products during the drying process, including other sugars, break down eventually and react with the products and damage them. But trehalose doesn’t do that.’ It’s also inert in the body. It’s already being used as the stabilising agent in more than 25 pharmaceuticals.
In Stablepharma’s system, called StablevaX, the manufacturer’s liquid vaccine is added to a proprietary buffer containing the sugar and the solution loaded into a sponge. The wet sponge goes into a syringe and is dried. Unlike other sugars, trehalose doesn’t crystalise but gets gradually more viscous until it becomes a clear solid.
‘The product, including the molecules that could gradually damage it in storage, like oxygen or other chemicals, is trapped in a completely inert, transparent, soluble glass,’ Roser explains. ‘You’ve stopped chemistry … and everything is frozen in time and in space. When you add water, the whole process just goes into reverse, and the molecule ends up as it was when you started.’

Roser’s company has worked on around 90 vaccines that can be stabilised using this technique, and is now looking at those for coronavirus. He’s confident that mRNA can also be stabilised, but more challenging will be the lipid nanoparticles that surround it. However, his company is talking to the vaccine manufacturers about what could potentially be a second generation of vaccines against Covid-19.
While all eyes are on Covid-19, millions of children suffer from vaccine-preventable diseases because of the challenges of keeping them cold on the final leg of the journey. Médecins Sans Frontières estimates that 1.5 million under-5s die every year as a result. Asel Sartbaeva, a researcher in the Centre for Sustainable and Circular Technologies at the University of Bath, UK, has been working on creating heat stable formulations of vaccines such as a combined one against diphtheria, tetanus and whooping cough.
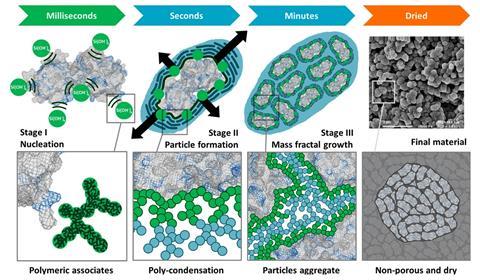
The approach she and her colleagues at Newcastle University have taken is to put the protein molecules of the vaccine into a protective silica cage, that is vacuum filtered and dried. They call the process ensilication. Using a solution–gelation method ‘we’re basically forcing our silica to break down beforehand. We pre- hydrolyse it and then, when we mix it with the protein, we force it to grow in a network all around the protein to encase it,’ explains Sartbaeva.
In their most recent study, the researchers showed that ensilication protected the tetanus vaccine – either stored at room temperature, transported without cooling, or heated to 80°C for two hours – so that it still produced an immune response in mice subsequently injected with it.2 The next steps are to look at encasing all three of the components of the DTP vaccine so it can be given in one injection as it is today. But the process needs to be better controlled, so the silica layering is consistent. The researchers also need a clinic-friendly mechanism to release the vaccine from its protective shell. At the moment the powder is put in a buffer solution to break down the silica shell, and the silica and protein separated. ‘It’s quite an easy method to use in the lab, but in a medical setting it would be hard.’ Work with colleagues in microfluidics is underway, with the aim of designing a device that will enable the vaccine to be released inside a syringe.
The urgency of tackling Covid-19 may have come too early for these advances, but their developers hope they’ll eventually help to put vaccines on shelves around the world for use whenever they’re needed.
References
1 L Bui-Le, APS Brogan and JP Hallett, Biotech. Bioeng., 2020, DOI: 10.1002/bit.27587
2 A Doekhie et al, Sci. Rep., 2020, 10, 9243, DOI: 10.1038/s41598-020-65876-3








1 Reader's comment