Chemical reaction flipped back and forth under scanning probe microscope
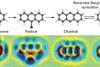
Work demonstrated on Bergman cyclisation offers route to valuable new reactions

Work demonstrated on Bergman cyclisation offers route to valuable new reactions