Chiral borane complexes catalyse new synthesis opportunities
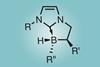
N-heterocyclic carbene-borane complexes with a rare stereogenic boron centre demonstrate potential in stereoselective catalysis
Researchers have developed a library of chiral N-heterocyclic carbene (NHC)-boranes that are stereogenic at the boron atom. These complexes are formed in an excellent diastereomeric ratio without the need for chiral separation, and may lead to new stereoselective catalysts.